Session Information
Date: Tuesday, October 28, 2025
Title: (1855–1876) Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: BAG3 (Bcl2-associated athanogene 3) regulates cellular pathways including apoptosis and autophagy, and induces fibroblast-to-myofibroblast transformation. In systemic sclerosis (SSc), myofibroblasts drive fibrosis of skin and internal organs, with interstitial lung disease (ILD) affecting ~50% of patients and being the leading cause of mortality. Recent evidence shows BAG3 serum levels correlate with nintedanib treatment response in SSc-ILD. This study aimed to investigate the role of BAG3 in SSc pathophysiology using complementary in vitro, ex vivo, and in vivo approaches
Methods: Immunohistochemistry of BAG3 expression was performed on lung tissue from SSc patients (Nf3) and healthy controls (Nf5). For in vivo studies, pulmonary fibrosis was induced in mice (Nf23) using bleomycin, followed by treatment with either vehicle (Nf11) or anti-BAG3 mAb (20mg/Kg twice weekly, Nf12) after 7 days until day 28. Lungs were collected for proteomic analysis using label-free shotgun proteomics following the SP3 protocol, with protein expression analyzed using Proteome Discoverer 2.4 software. Skin-derived fibroblasts of 10 SSc patients and 7 healthy controls (HC) were evaluated for BAG3 expression and its receptor. We tested nintedanib’s (50nM) influence on BAG3 release in HC fibroblasts before and after TGFβ stimulation.
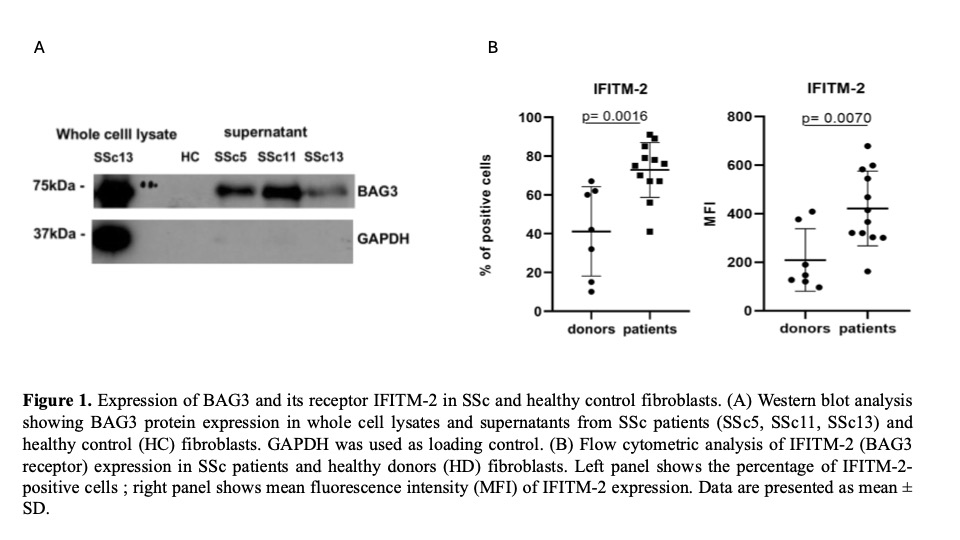
Results: Our study revealed a clear pattern of BAG3 dysregulation in SSc. Western blotting demonstrated significantly higher BAG3 expression in SSc skin fibroblasts versus HC, both in whole cell lysates and supernatants (Fig 1A), while flow cytometry showed corresponding upregulation of the BAG3 receptor IFITM-2 in SSc fibroblasts (70% positive cells vs 40% in HC, p=0.0016), with increased mean fluorescence intensity (p=0.007) indicating higher receptor density (Fig 1B). To understand the regulation of this pathway, we assesed that TGF-β1, a key profibrotic cytokine, significantly induced BAG3 expression in HC fibroblasts in a dose-dependent manner (75 pg/ml at 10 ng/ml and 40 pg/ml at 5 ng/ml TGFβ, p< 0.01). Importantly, nintedanib treatment effectively suppressed this TGF-β-induced BAG3 expression to near-baseline levels (Fig 2), suggesting a mechanism for its antifibrotic effects. Extending these findings to tissue level, immunohistochemistry confirmed significant BAG3 overexpression in SSc lung specimens compared to controls, particularly in areas with inflammatory infiltrates and fibrotic changes. To establish causality, we employed a bleomycin-induced pulmonary fibrosis model where anti-BAG3 mAb treatment (Nf12) markedly reduced fibrotic areas compared to PBS-treated controls (Nf11). Proteomic analysis of lung tissue from both experimental groups revealed that BAG3 inhibition downregulated key pathological processes including apoptosis, fibrosis, and inflammation while activating cell survival pathways, providing mechanistic insights into BAG3's role in fibrosis pathogenesis.
Conclusion: This study establishes BAG3 as a central player in SSc-associated fibrosis. These findings identify BAG3 as both a potential therapeutic target and biomarker for monitoring treatment response in SSc-ILD, warranting further investigation in larger cohorts.
To cite this abstract in AMA style:
Iannone C, De Marco M, Minniti A, Armentaro G, Falco A, D'Ardia A, Manzo p, Marzullo L, Basile A, Rosati A, Turco M, Caporali R, Del Papa N. Bag3 in systemic sclerosis: possible therapeutic target and biomarker for pulmonary fibrosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/bag3-in-systemic-sclerosis-possible-therapeutic-target-and-biomarker-for-pulmonary-fibrosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bag3-in-systemic-sclerosis-possible-therapeutic-target-and-biomarker-for-pulmonary-fibrosis/

.jpg)