Session Information
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: One proposed mechanism of tissue damage in ANCA-associated vasculitis (AAV) is the formation of neutrophil extracellular traps (NETs) during extravasation of neutrophils into and through blood vessel walls. NETs carry antimicrobial peptides, myeloperoxidase, proteinase 3, and alternative pathway (AP) complement protein split products. AAV is typically described as pauci-immune, with renal and other tissues often reported as C3 negative.This study aimed to characterize complement activation in affected tissue in AAV using novel techniques to identify active complement as compared to C3d deposition. C3d is a covalently membrane-bound biomarker generated by C3 cleavage that demonstrates extended tissue half-life.
Methods: Immunofluorescence (IF) staining was performed using an anti-C3c polyclonal antibody (Dako) and a novel anti-C3d monoclonal antibody (mAb, 3d8b mouse IgG1). IF was conducted on renal biopsy samples from patients with AAV (n=32), IgA nephropathy (IgAN, n=29), C3 glomerulonephritis (C3G, n=9), and class III lupus nephritis (LN, n=30), and skin biopsy samples from patients with leukocytoclastic vasculitis (LCV, n=37), and skin lesions in AAV (n=4). Immunohistochemistry (IHC) staining of C3d was performed on biopsy samples of kidney (n=8), skin (n=3), lung (n=5), and sinus (n=2) from patients with AAV. Immunostainings (IF and IHC) were semi-quantitatively scored. Renal biopsy IF intensity was further evaluated for digital quantification using image analysis software.
Results: IF staining detected active complement (anti-C3c) and C3d deposition (anti-C3d) in the glomeruli of AAV, IgAN, C3G, and LN) (Figure 1). Both semi-quantitative scoring and digital quantification revealed a moderate glomerular IF intensity in AAV and IgAN, and a higher intensity in C3G and LN. A distinct glomerular staining pattern with C3c/C3d was seen in AAV and IgAN with deposition more in mesangium than capillaries, as compared to C3G and LN in which C3c/C3d deposited equally in mesangium and capillaries. IHC staining of AAV renal tissue confirmed C3d deposition in glomeruli and revealed extra-glomerular small vessels (Figure 2); 3/6 of these same biopsies tested negative for C3 by routine diagnostic immunostaining. IF staining detected active complement and C3d deposition on superficial dermal vasculature in LCV. Semi-quantitative scoring demonstrated higher anti-C3c staining than anti-C3d IF intensity in four AAV-LCV samples, as compared to non AAV-LCV samples (Figure 3). IHC staining of FFPE tissue also detected C3d deposition in skin, lung, and sinus biopsies from AAV patients.
Conclusion: Immunostaining for complement AP fragments, particularly C3d, reveals evidence of complement activation in AAV in kidney, skin, lung, and sinus, including biopsies deemed negative by routine C3 staining. These data demonstrate that glomerulonephritis in AAV is less “pauci-immune” than previously considered. These findings also suggest that the complement AP contributes to the pathogenesis of AAV and that treatment using an anti-C3d tissue targeted AP inhibitor approach could mitigate inflammation-related damage in AAV.
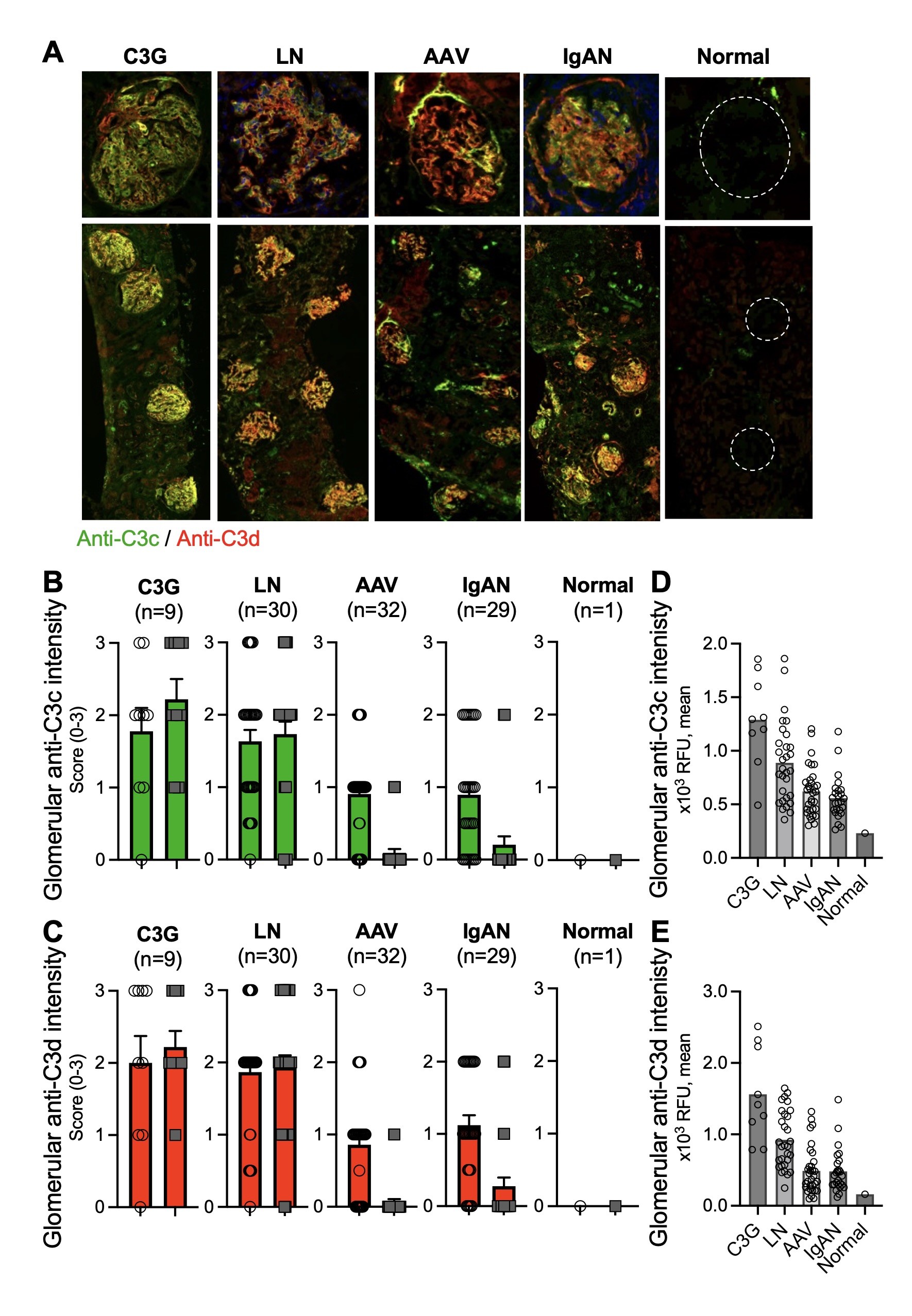 Figure 1. Immunofluorescent staining developed to capture complement fragments demonstrates glomerular complement activation in frozen kidney biopsies from patients with ANCA-associated vasculitis, in comparison to other glomerulonephritides and normal tissue. Anti-C3c and anti-C3d stainings (A, representative images) were semi-quantitatively scored (B-C) and evaluated for digital quantification (D-E).
Figure 1. Immunofluorescent staining developed to capture complement fragments demonstrates glomerular complement activation in frozen kidney biopsies from patients with ANCA-associated vasculitis, in comparison to other glomerulonephritides and normal tissue. Anti-C3c and anti-C3d stainings (A, representative images) were semi-quantitatively scored (B-C) and evaluated for digital quantification (D-E).
.jpg) Figure 2. Histochemistry staining reveals the presence of C3d deposition in extra-glomerular kidney small vessels, in formalin-fixed-paraffin-preserved kidney biopsies from patients with ANCA-associated vasculitis.
Figure 2. Histochemistry staining reveals the presence of C3d deposition in extra-glomerular kidney small vessels, in formalin-fixed-paraffin-preserved kidney biopsies from patients with ANCA-associated vasculitis.
.jpg) Figure 3. Immunofluorescent staining in lesional skin biopsies (A, representative images) reveals a higher level of complement activation in biopsy specimens of leukocytoclastic vasculitis from patients with ANCA-associated vasculitis (AAV-LCV) compared to samples from patients without AAV (Non AAV-LCV) (B).
Figure 3. Immunofluorescent staining in lesional skin biopsies (A, representative images) reveals a higher level of complement activation in biopsy specimens of leukocytoclastic vasculitis from patients with ANCA-associated vasculitis (AAV-LCV) compared to samples from patients without AAV (Non AAV-LCV) (B).
To cite this abstract in AMA style:
Galand C, Liu F, Vernon K, Vats R, Lian C, Holers V, Cook H, Cuthbertson D, Khalidi N, Koening C, Langford C, McAlear C, Monach P, Pagnoux C, Campagna J, Violette S, Merkel P. Identification of C3 complement fragments in lesional tissues in ANCA-associated vasculitis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/identification-of-c3-complement-fragments-in-lesional-tissues-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-c3-complement-fragments-in-lesional-tissues-in-anca-associated-vasculitis/
