Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Basic Science (1752–1757)
Session Type: Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: The rs6759298 variant near B3GNT2 is associated with Axial Spondyloarthritis (AS); however, its pathogenic mechanisms remain unknown. In this study, we explored the role of B3GNT2 in glycosylation modifications and their impact on Th17/Treg imbalance, contributing to AS development.
Methods: We conducted SNP genotyping and RNA-seq analysis on blood samples from 39 AS patients. Additionally, we generated B3gnt2-deficient mice (B3gnt2+/- and B3gnt2-/-) to investigate the role of B3GNT2 in AS using a proteoglycan-induced arthritis (PGIA) model. The mechanism of B3GNT2 in AS was further explored through flow cytometry, RNA-seq, lectin blotting and mass spectrometry analysis.
Results: The rs6759298 mutant genotype (G/G) significantly downregulated B3GNT2 expression in AS patients. In the PGIA model, B3gnt2-deficient mice exhibited an earlier onset of arthritis, higher arthritis severity scores, and prolonged inflammation. Functionally, we found a disrupted Th17/Treg balance in B3gnt2-deficient mice. Lectin blotting analysis revealed a marker reduction in poly-lactosamine expression on T cells from B3gnt2-deficient mice. Mass spectrometry identified integrin β3 (ITGB3) as a key glycoprotein differentially modified by B3GNT2. Furthermore, treatment of SKG mice with Cilengitide, an ITGB3 antagonist, effectively delayed disease onset.
Conclusion: This study demonstrates that rs6759298 reduces B3GNT2 gene expression, thereby altering the glycosylation of ITGB3 and promoting the progression of AS. These findings offer new insights into the immune mechanisms underlying AS.
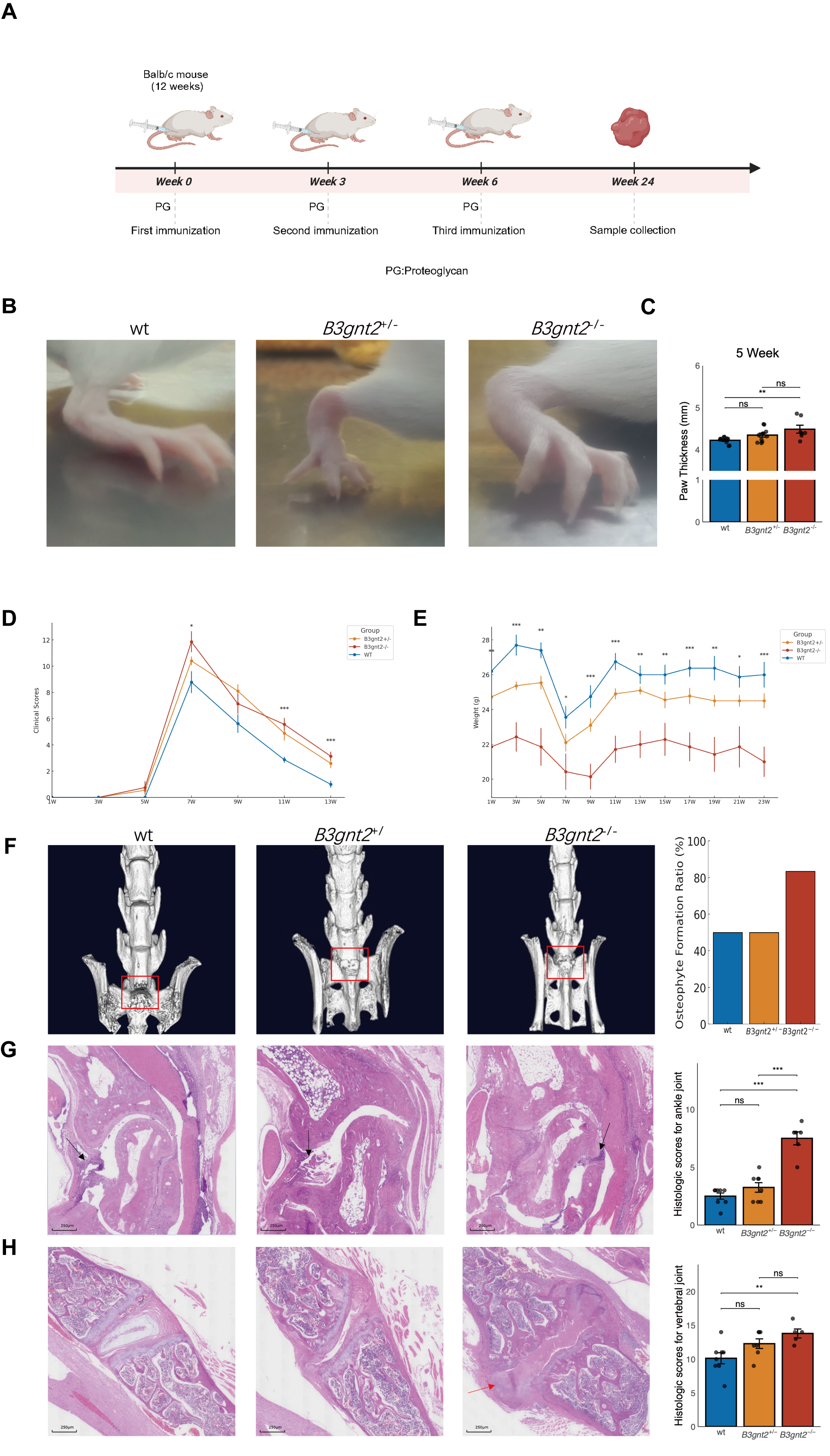 Figure 1. B3gnt2 Knockout Promotes inflammation and exacerbated osteophyte formation in Proteoglycan-induced arthritis Mice. (A) Schematic diagram for study design. Created in BioRender. (B) Representative pictures of the paw in three groups of mice at week 5. (C)Thickness of ankle joint in three groups of mice at week 5. (D) Degree of paw swelling in three groups of mice (n = 10). (E) Body weight monitoring in model mice. (F) Representative pictures of Micro-CT of the sacroiliac joint in three groups of mice at the end of week 24 and Statistics of osteophyte formation rate. Osteophyte formation (red square). (G) Representative pictures of ankle joint sections in three groups of mice (HE staining, scale bar = 250 µm) and histologic scores for ankle joint sections. Inflammatory cell infiltration in the synovium of the joint capsule (black arrow). (H) Representative pictures of vertebral joint sections in three groups of mice (HE staining, scale bar = 250 µm) and histologic scores of vertebral joint sections. New cartilage (red arrow). *P < 0.05, **P < 0.01, ***P < 0.001; ns, Not Significant.
Figure 1. B3gnt2 Knockout Promotes inflammation and exacerbated osteophyte formation in Proteoglycan-induced arthritis Mice. (A) Schematic diagram for study design. Created in BioRender. (B) Representative pictures of the paw in three groups of mice at week 5. (C)Thickness of ankle joint in three groups of mice at week 5. (D) Degree of paw swelling in three groups of mice (n = 10). (E) Body weight monitoring in model mice. (F) Representative pictures of Micro-CT of the sacroiliac joint in three groups of mice at the end of week 24 and Statistics of osteophyte formation rate. Osteophyte formation (red square). (G) Representative pictures of ankle joint sections in three groups of mice (HE staining, scale bar = 250 µm) and histologic scores for ankle joint sections. Inflammatory cell infiltration in the synovium of the joint capsule (black arrow). (H) Representative pictures of vertebral joint sections in three groups of mice (HE staining, scale bar = 250 µm) and histologic scores of vertebral joint sections. New cartilage (red arrow). *P < 0.05, **P < 0.01, ***P < 0.001; ns, Not Significant.
.jpg) Figure 2. B3gnt2 knockout resulted in Th17/Treg imbalance. (A) Gating strategy used for analysis of Th cells. Arrows indicate that Th cells were sequentially gated from single cells, CD45+ cells, CD3+ cells and CD4+ cells. Gated CD4+ cells were analyzed for expression of IL-17A, IFN-γ, and IL-4. (B) Percentage of IL-17A+ cells or IFN-γ+ cells or IL-4+cells among CD4+ T cells in PBMC. (C) Cell ratio in spleen. (D) Cell ratio in Intestine. (E) Gating strategy used for analysis of Treg cells. Arrows indicate that Treg cells were sequentially gated from single cells, CD45+ cells, CD3+ cells and CD4+ cells. Gated CD4+ cells were analyzed for expression of CD25 and FoxP3. (F) Percentage of CD25+ FoxP3+ cells among CD4+ T cells in PBMC. (G) Cell ratio (Th17/Treg in PBMC). *P < 0.05, **P < 0.01, ***P < 0.001; ns, Not Significant.
Figure 2. B3gnt2 knockout resulted in Th17/Treg imbalance. (A) Gating strategy used for analysis of Th cells. Arrows indicate that Th cells were sequentially gated from single cells, CD45+ cells, CD3+ cells and CD4+ cells. Gated CD4+ cells were analyzed for expression of IL-17A, IFN-γ, and IL-4. (B) Percentage of IL-17A+ cells or IFN-γ+ cells or IL-4+cells among CD4+ T cells in PBMC. (C) Cell ratio in spleen. (D) Cell ratio in Intestine. (E) Gating strategy used for analysis of Treg cells. Arrows indicate that Treg cells were sequentially gated from single cells, CD45+ cells, CD3+ cells and CD4+ cells. Gated CD4+ cells were analyzed for expression of CD25 and FoxP3. (F) Percentage of CD25+ FoxP3+ cells among CD4+ T cells in PBMC. (G) Cell ratio (Th17/Treg in PBMC). *P < 0.05, **P < 0.01, ***P < 0.001; ns, Not Significant.
.jpg) Figure 3. B3GNT2 Targets ITGB3 as a Key Glycosylation Substrate. (A) Schematic diagram for study design. Created in BioRender. (B) Lysates of T cells were incubated overnight and subjected to LEL blots for polylactosamine. (C) Lysates of Spleen cells were incubated overnight and subjected to LEL blots for polylactosamine. (D) Principal component analysis of protein across three groups (n=3/group). (E) Heatmap visualization of differentially expressed proteins with hierarchical clustering. (F) Volcano plot of differentially expressed proteins in B3gnt2-/- vs WT. (G) Gene Ontology enrichment of downregulated proteins in B3gnt2-/- vs WT.
Figure 3. B3GNT2 Targets ITGB3 as a Key Glycosylation Substrate. (A) Schematic diagram for study design. Created in BioRender. (B) Lysates of T cells were incubated overnight and subjected to LEL blots for polylactosamine. (C) Lysates of Spleen cells were incubated overnight and subjected to LEL blots for polylactosamine. (D) Principal component analysis of protein across three groups (n=3/group). (E) Heatmap visualization of differentially expressed proteins with hierarchical clustering. (F) Volcano plot of differentially expressed proteins in B3gnt2-/- vs WT. (G) Gene Ontology enrichment of downregulated proteins in B3gnt2-/- vs WT.
To cite this abstract in AMA style:
Geng C, Zhuo D, Wang J, Liu J. B3GNT2 Regulates Integrin β3 Glycosylation and Th17/Treg Imbalance in Axial Spondyloarthritis Pathogenesis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/b3gnt2-regulates-integrin-%ce%b23-glycosylation-and-th17-treg-imbalance-in-axial-spondyloarthritis-pathogenesis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/b3gnt2-regulates-integrin-%ce%b23-glycosylation-and-th17-treg-imbalance-in-axial-spondyloarthritis-pathogenesis/
