Session Information
Session Type: Abstract Session
Session Time: 11:00AM-11:15AM
Background/Purpose: Interstitial lung disease (ILD) is a serious pulmonary complication of rheumatoid arthritis (RA), contributing significantly to morbidity and mortality. Non-invasive tools for identifying ILD risk in RA patients remain limited. As a stable and accessible biofluid, urine represents a promising source of biomarkers. We aimed to identify ILD-associated urinary proteomic signatures and evaluate their potential utility for risk stratification and clinical assessment.
Methods: We analyzed the urine proteome of 78 RA patients (14 with ILD and 64 without ILD) using data-independent acquisition (DIA) liquid chromatography-tandem mass spectrometry (LC-MS). ILD-associated proteins were identified through differential expression analysis and LASSO regression, supported by 1000 bootstrap iterations. Candidate markers were evaluated using logistic regression, ROC analysis, and correlation with pulmonary function.
Results: A total of 2,482 urinary proteins were quantified. Differential expression analysis, LASSO regression, and 1,000 bootstrap iterations (Figure 1) jointly identified two robust ILD-associated proteins: SPOCK1 and PGRMC1, both significantly upregulated in RA-ILD patients (p < 0.001). A composite Urine_ILD_Score was constructed based on their expression levels. The score was significantly elevated in ILD patients (p < 0.001; Figure 2A) and associated with pulmonary impairment. In ROC analysis, the score demonstrated high discriminatory ability (AUC = 0.904, p < 0.0001), outperforming either protein alone (SPOCK1: AUC = 0.812; PGRMC1: AUC = 0.786; Figure 2D). In multivariable logistic regression adjusting for age and sex, the Urine_ILD_Score remained independently associated with ILD (OR = 6.19, 95% CI [2.34–24.64], p = 0.002; Figure 2C). A combined multivariable model incorporating clinical covariates further improved classification performance (AUC = 0.950; Figure 2D). Importantly, PGRMC1 levels correlated positively with KL-6 (r = 0.79, p = 0.028) and negatively with total lung capacity (TLC%) (r = –0.79, p = 0.048), suggesting potential relevance to ILD pathogenesis and severity.
Conclusion: Urinary proteomic profiling can identify RA patients at increased risk for ILD. A composite score based on SPOCK1 and PGRMC1 provides a promising, non-invasive biomarker for ILD risk stratification in RA and may support clinical decision-making and patient monitoring.
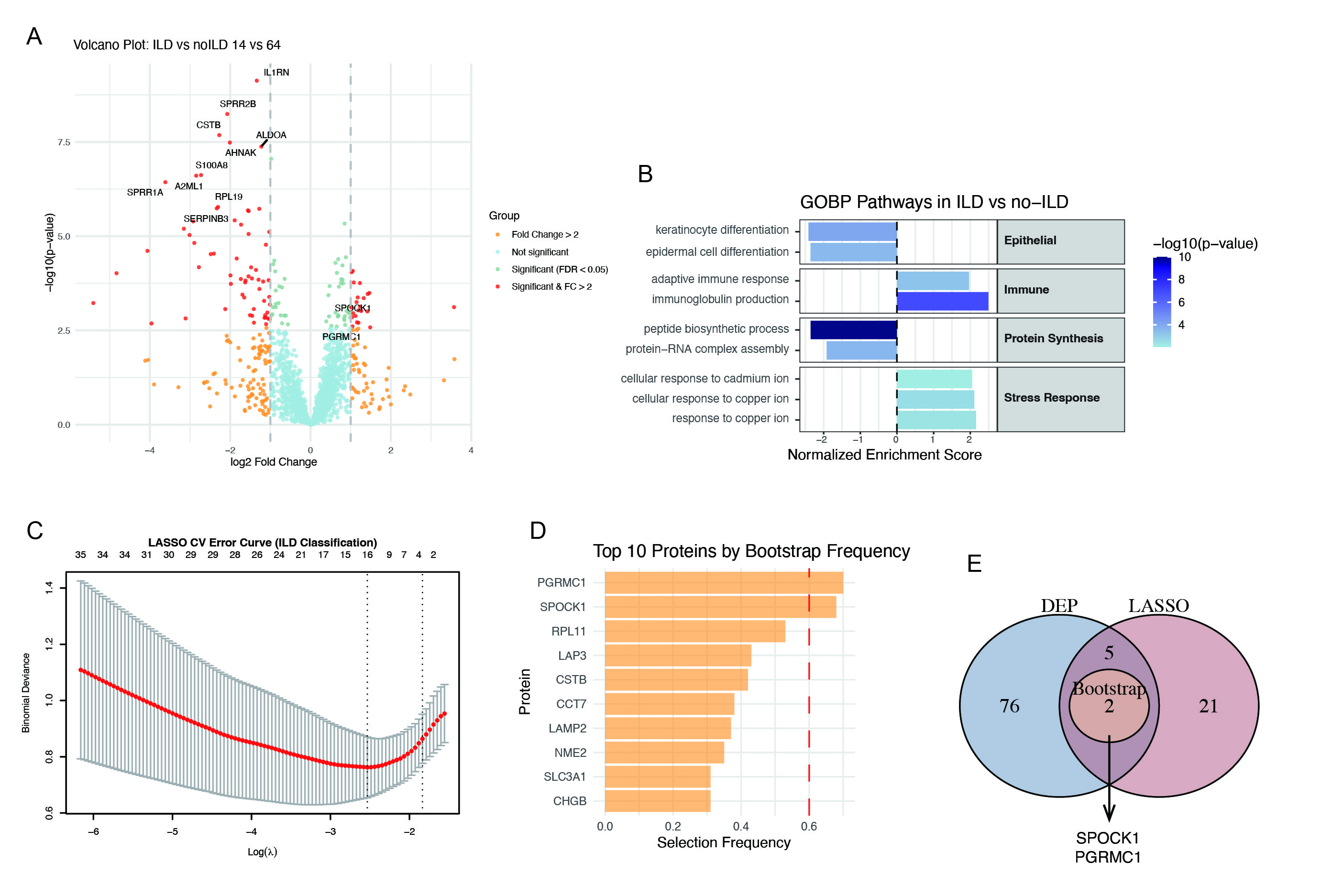 Figure 1. Identification of ILD-associated urinary proteins in RA patients.
Figure 1. Identification of ILD-associated urinary proteins in RA patients.
(A)Volcano plot of differentially expressed urinary proteins between RA patients with ILD (n = 14) and without ILD (n = 64).
(B) Gene set enrichment analysis (GSEA) of Gene Ontology Biological Processes based on ranked protein expression between ILD and no-ILD RA patients.
(C) LASSO regression with 10-fold cross-validation for ILD classification.
(D) Top 10 proteins ranked by bootstrap selection frequency across 1,000 iterations.
(E) Venn diagram showing overlap of differentially expressed proteins, LASSO-selected features, and proteins consistently selected by bootstrap.
.jpg) Figure 2. Urine-based protein biomarkers and composite score predict ILD in RA patients.
Figure 2. Urine-based protein biomarkers and composite score predict ILD in RA patients.
(A)Expression levels of SPOCK1, PGRMC1, and the composite Urine_ILD_Score in RA patients with and without ILD.
(B) Forest plot of univariable logistic regression assessing the association of clinical and proteomic variables with ILD status.
(C) Forest plot of multivariable logistic regression including age and sex as covariates.
(D) ROC analysis comparing the discriminatory performance of SPOCK1, PGRMC1, the composite score, and a full multivariable model (including clinical covariates).
To cite this abstract in AMA style:
Shi J, Yuan X, Deng Y, Yu C, Jiang N, Guo Z, Lood C, Li M, Sun W, Wang Q, Tian X. Urinary Proteomic Signature Identifies Rheumatoid Arthritis Patients at Risk for Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/urinary-proteomic-signature-identifies-rheumatoid-arthritis-patients-at-risk-for-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/urinary-proteomic-signature-identifies-rheumatoid-arthritis-patients-at-risk-for-interstitial-lung-disease/
