Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Immunological Complications of Medical Therapy (1728–1733)
Session Type: Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: As immune checkpoint inhibitor (ICI) usage increases for various cancers the prevalence of rheumatologic immune related adverse events (irAEs) also grows. Glucocorticoids are first line treatment, but standardization in dosing, tapering, and duration is lacking. There are mixed results on the effect of oral glucocorticoids on progression-free survival (PFS) among ICI-treated patients. We investigated oral glucocorticoid use and its association with PFS among ICI-treated cancer patients with ICI-inflamatory arthritis (ICI-IA).
Methods: Data from the RADIOS registry, a prospective U.S. multi-center rheumatic irAE cohort was used. Patients enrolled since February 2023 who were treated with an ICI for cancer and who developed ICI-IA, which we defined as inflammatory arthritis, arthralgia, or PMR, and were treated with glucocorticoids for ICI-IA were included. Patients were excluded if they had pre-existing autoimmune disease or were being treated with glucocorticoids for another irAE. Demographics, cancer and cancer treatment, DMARD and glucocorticoid treatment were collected. Glucocorticoid dosage was converted to prednisone equivalents. Cumulative glucocorticoid exposure and the average daily prednisone dose was calculated at 1-2 weeks, 2-4 weeks, month 2, and months 3,4,5 and displayed in a box plot. Descriptive statistics were calculated. Thirty-day landmark Kaplan Meier plots assessed glucocorticoid exposure and its association with PFS, measured from glucocorticoid initiation to radiographic cancer progression or death. Adjusted cox proportional models with a time varying covariant (time from ICI initiation to glucocorticoid treatment) were performed. Models were adjusted for age, irAE grade at baseline, cancer type (melanoma, non-small cell lung cancer [NSCLC], renal cell cancer [RCC]), cancer stage, ICI combination therapy.
Results: The analytic cohort consisted of 206 patients with a mean age of 65 (SD 12.36), 48.5% were female and 85.9% were White (Table 1). Most patients had melanoma (32.5%), RCC (18.4%), or NSCLC (11.7%), and were stage 3 (26.7%) or 4 (58.3%). The median time from ICI-IA diagnosis to glucocorticoid initiation was 24.5 days (IQR 3, 63). Cancer progression occurred in 46 patients (22.3%), and the median time from glucocorticoid initiation to cancer progression was 82.5 days (IQR -70, 283).Figure 1a displays the median and interquartile ranges of the average daily prednisone dose, which was highest in the first month of treatment and decreased over time. The first month median glucocorticoid dose was not associated with PFS in a landmark Kaplan Meier curve (log-rank p-value 0.99, Figure 1b). Additionally, there was no difference between quartiles of glucocorticoid dose and PFS (log rank p-value 0.31, Figure 1c). None of the adjusted Cox models were significant for glucocorticoid dose and PFS.
Conclusion: In this cohort, there was not a dose-dependent effect of glucocorticoids on PFS in patients with ICI-IA. Rheumatologists typically use a lower dose than oncology guidelines suggest, and these findings suggest that glucocorticoid treatment of ICI-IA may not have a substantial impact on cancer outcomes.
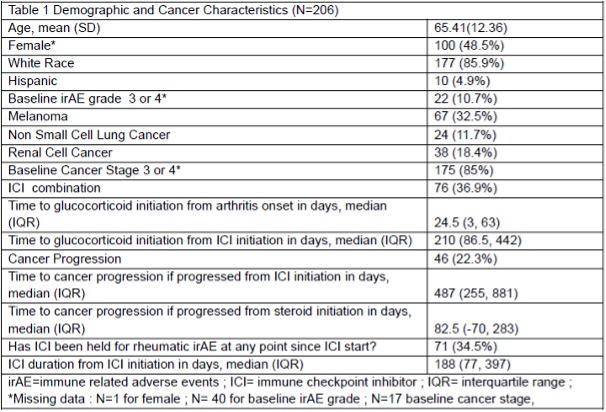 Table1. Demographic and Cancer Characteristics of Nf206 RADIOS patients
Table1. Demographic and Cancer Characteristics of Nf206 RADIOS patients
.jpg) Figure 1. A) Box plot of average daily prednisone doses by time period. B) 30 Day Landmark Kaplan Meier Curve by population median steroid dose C) 30 day Landmark Kaplan Meier Curve by quartiles of steroid dose
Figure 1. A) Box plot of average daily prednisone doses by time period. B) 30 Day Landmark Kaplan Meier Curve by population median steroid dose C) 30 day Landmark Kaplan Meier Curve by quartiles of steroid dose
To cite this abstract in AMA style:
Jannat-Khah D, Reid P, Suarez-Almazor M, Abdel-Wahab N, Sparks J, Braaten T, Calabrese C, Meara A, Nong M, Ge K, Cappelli L, Shah A, Bingham C, Bass A. Oral glucocorticoid treatment for checkpoint inhibitor associated inflammatory arthritis do not affect progression free survival: a RADIOS Registry cohort study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/oral-glucocorticoid-treatment-for-checkpoint-inhibitor-associated-inflammatory-arthritis-do-not-affect-progression-free-survival-a-radios-registry-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/oral-glucocorticoid-treatment-for-checkpoint-inhibitor-associated-inflammatory-arthritis-do-not-affect-progression-free-survival-a-radios-registry-cohort-study/
