Session Information
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: Type I interferons (IFN-I) and type II interferon (IFN-g) are essential to host defense but dysregulated production of these cytokines is increasingly recognized in inflammatory diseases. Aberrant IFN-I production is associated with autoimmunity and excess IFN-g is linked to cytokine storm syndromes with T lymphocyte activation. Assays to measure IFN-I and IFN-g are not available in most institutions. We aimed to develop a clinical flow cytometry assay that rapidly captures the activity of IFN-I and IFN-g.
Methods: We compared bulk RNA sequencing data from healthy controls and patients with macrophage activation syndrome (MAS), multisystem inflammatory syndrome in children (MIS-C), and systemic lupus erythematosus (SLE) to identify biomarkers of IFN-I and IFN-g signaling. We developed a flow cytometry assay to measure IFN-inducible markers on peripheral blood monocytes and acquired data across different pediatric inflammatory diseases. The findings were verified by a clinical laboratory that independently developed and validated this assay for clinical use.
Results: Consistent with previous studies, RNA sequencing data from peripheral blood mononuclear cells identified a strong IFN-I signature in patients with SLE and a strong IFN-g signature in patients with MAS and MIS-C. We found that CD169 (SIGLEC-1) transcript levels positively correlated with the IFN-I signature while CD274 (PD-L1) expression reflected the IFN-g signature (Figure 1). Flow cytometry confirmed that surface expression of CD169 and CD274 on CD14+ monocytes are specific readouts of IFN-I and IFN-g activity, respectively (Figure 2). Based on these findings, we developed a flow cytometry assay to capture CD169 and CD274 expression, which requires 50 µL of whole blood and 30 minutes of hands-on time. We demonstrated the utility of this method for rapid screening of IFN dysregulation in >200 patients with a variety of pediatric inflammatory diseases including MAS, MIS-C, SLE, juvenile idiopathic arthritis, juvenile dermatomyositis, and monogenic autoinflammatory diseases (Figure 3A-D). We further illustrated the use of this test for therapeutic monitoring of recently approved medications that target the IFN pathways including Janus kinase inhibitors, emapalumab (anti-IFN-g), and anifrolumab (anti-IFN α/β receptor; Figure 3E-G). Finally, we outlined the steps of validating this assay for clinical use and summarize the findings during test validation and post-clinical implementation.
Conclusion: We developed a clinically validated flow cytometry test to rapidly assess type I IFN and IFN-g activity. This test is useful for evaluation and monitoring of pediatric inflammatory diseases and can be readily implemented in clinical laboratories.
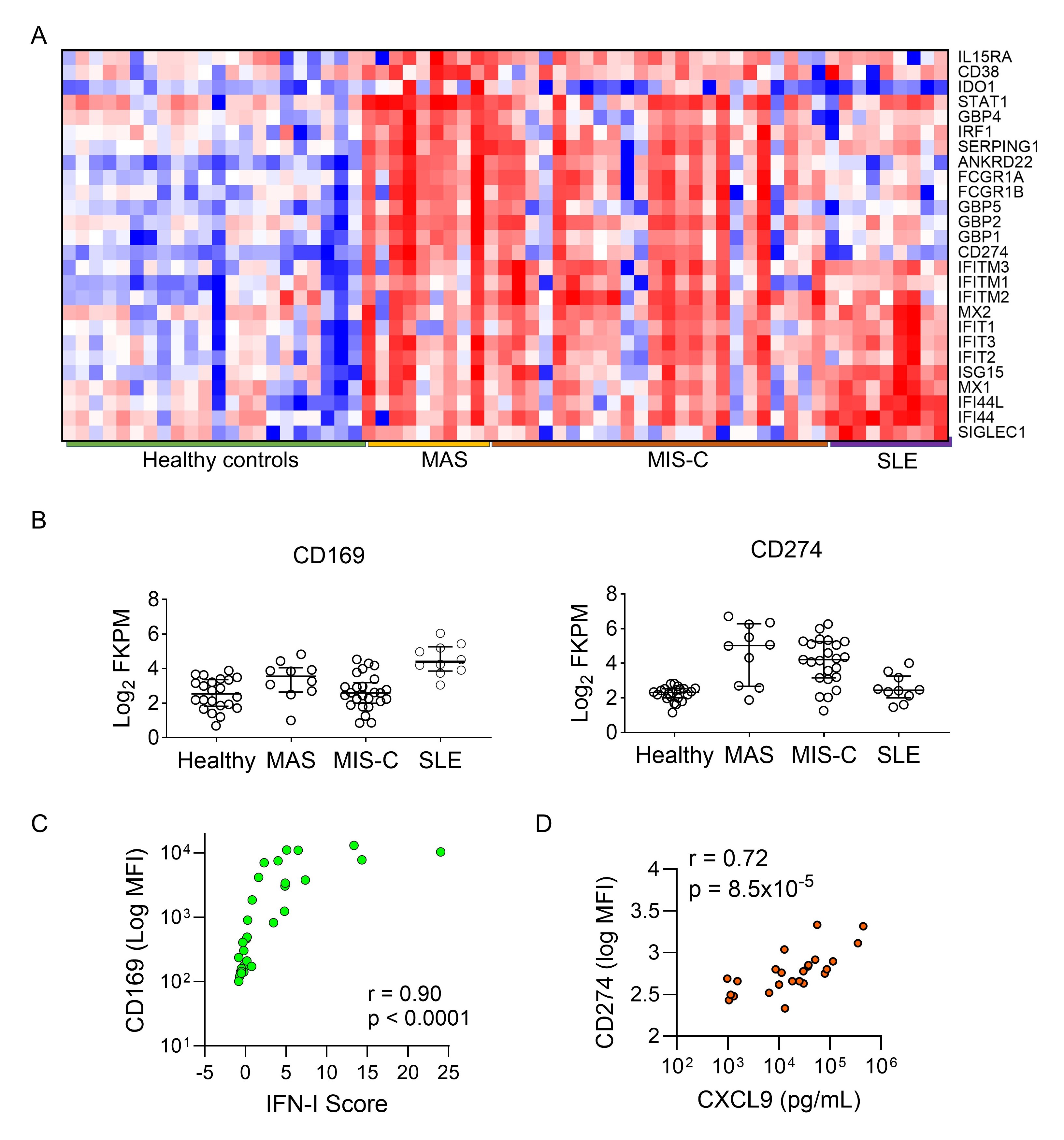 Figure 1. Bulk RNA sequencing data of peripheral blood mononuclear cells from healthy controls and patients with MAS, MIS-C, or SLE. A) Heat map comparing the expression of genes preferentially induced by IFN- (top half) and by IFN-I (bottom half). B) Comparison of CD169 (SIGLEC-1) and CD274 (PD-L1) expression among healthy controls and patient groups. C) Spearman’s correlation between CD169 expression and the 28-gene composite IFN-I score in healthy controls and patients with SLE. D) Spearman’s correlation between CD274 expression and plasma CXCL9 levels in patients with MAS.
Figure 1. Bulk RNA sequencing data of peripheral blood mononuclear cells from healthy controls and patients with MAS, MIS-C, or SLE. A) Heat map comparing the expression of genes preferentially induced by IFN- (top half) and by IFN-I (bottom half). B) Comparison of CD169 (SIGLEC-1) and CD274 (PD-L1) expression among healthy controls and patient groups. C) Spearman’s correlation between CD169 expression and the 28-gene composite IFN-I score in healthy controls and patients with SLE. D) Spearman’s correlation between CD274 expression and plasma CXCL9 levels in patients with MAS.
.jpg) Figure 2. Development of a flow cytometry assay to evaluate IFN activation. A) Gating strategy and representative histograms for evaluation of CD169 and CD274 expression in peripheral blood CD14+ monocytes from a healthy donor with and without IFN stimulation. B) Change in mean fluorescence intensity of CD274 and CD169 expression in response to IFN stimulation at different concentrations. C) Changes in CD169 and CD274 expression on CD14+ monocytes from 4 healthy donors in response to stimulation by different cytokines.
Figure 2. Development of a flow cytometry assay to evaluate IFN activation. A) Gating strategy and representative histograms for evaluation of CD169 and CD274 expression in peripheral blood CD14+ monocytes from a healthy donor with and without IFN stimulation. B) Change in mean fluorescence intensity of CD274 and CD169 expression in response to IFN stimulation at different concentrations. C) Changes in CD169 and CD274 expression on CD14+ monocytes from 4 healthy donors in response to stimulation by different cytokines.
.jpg) Quantifying IFN-I and IFN- activity in pediatric inflammatory diseases. A) CD169 and B) CD274 expression in healthy controls compared to patients with different inflammatory diseases. C) CD169 and D) CD274 expression in healthy controls compared to patients with familial hemophagocytic lymphohistiocytosis and monogenic autoinflammatory diseases. E) Changes in CD169 expression in patients with Aicardi-Goutières syndrome after treatment with JAK inhibitors. F) Changes in CD169 expression in a patient with SLE and a patient with Singleton-Merten syndrome after anifrolumab treatment. G) Changes in CD274 expression in patients with MAS after initiation of emapalumab. Green area indicates normal range (mean +/- 2 standard deviations) established by the healthy control group.
Quantifying IFN-I and IFN- activity in pediatric inflammatory diseases. A) CD169 and B) CD274 expression in healthy controls compared to patients with different inflammatory diseases. C) CD169 and D) CD274 expression in healthy controls compared to patients with familial hemophagocytic lymphohistiocytosis and monogenic autoinflammatory diseases. E) Changes in CD169 expression in patients with Aicardi-Goutières syndrome after treatment with JAK inhibitors. F) Changes in CD169 expression in a patient with SLE and a patient with Singleton-Merten syndrome after anifrolumab treatment. G) Changes in CD274 expression in patients with MAS after initiation of emapalumab. Green area indicates normal range (mean +/- 2 standard deviations) established by the healthy control group.
To cite this abstract in AMA style:
Hsu E, Leson C, Basu A, Lam M, Yue J, Rimland C, Weng R, Henderson L, Chang J, Son M, Dedeoglu F, Abraham r, Lee P. A clinically validated assay for rapid determination of type I and type II interferon activity in pediatric inflammatory diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-clinically-validated-assay-for-rapid-determination-of-type-i-and-type-ii-interferon-activity-in-pediatric-inflammatory-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-clinically-validated-assay-for-rapid-determination-of-type-i-and-type-ii-interferon-activity-in-pediatric-inflammatory-diseases/
