Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases II: Models and Mechanisms (1662–1667)
Session Type: Abstract Session
Session Time: 1:45PM-2:00PM
Background/Purpose: Uveitis is a vision-threatening manifestation of systemic rheumatic diseases. While conventional immunosuppressants are typically first-line, biologics are often reserved for refractory cases. Long-term real-world data comparing these approaches remain limited.
Methods: We performed a retrospective cohort study using TriNetX, a global database of de-identified electronic health records from 146 healthcare organizations. Adults with non-infectious uveitis and a systemic rheumatic disease were identified using ICD-10-CM codes. Patients were grouped into:(1) a conventional therapy cohort (corticosteroids plus methotrexate, azathioprine, or mycophenolate), and(2) a biologic therapy cohort (TNF, IL-17/23, or JAK inhibitors).Patients with infectious uveitis or concurrent use of both therapies were excluded. Among 11,432 eligible patients (6,661 in the conventional cohort; 4,771 in the biologic cohort), 2,985 from each group were matched 1:1 using propensity scores based on demographics, comorbidities, and baseline corticosteroid use. Outcomes were assessed at 1 and 5 years following the index treatment. Primary endpoints included ocular complications, ophthalmic procedures, systemic infections, cardiovascular events, and all-cause mortality. Risk ratios (RRs), 95% confidence intervals (CIs), and p-values were reported. A subgroup analysis compared TNF vs. JAK inhibitors.
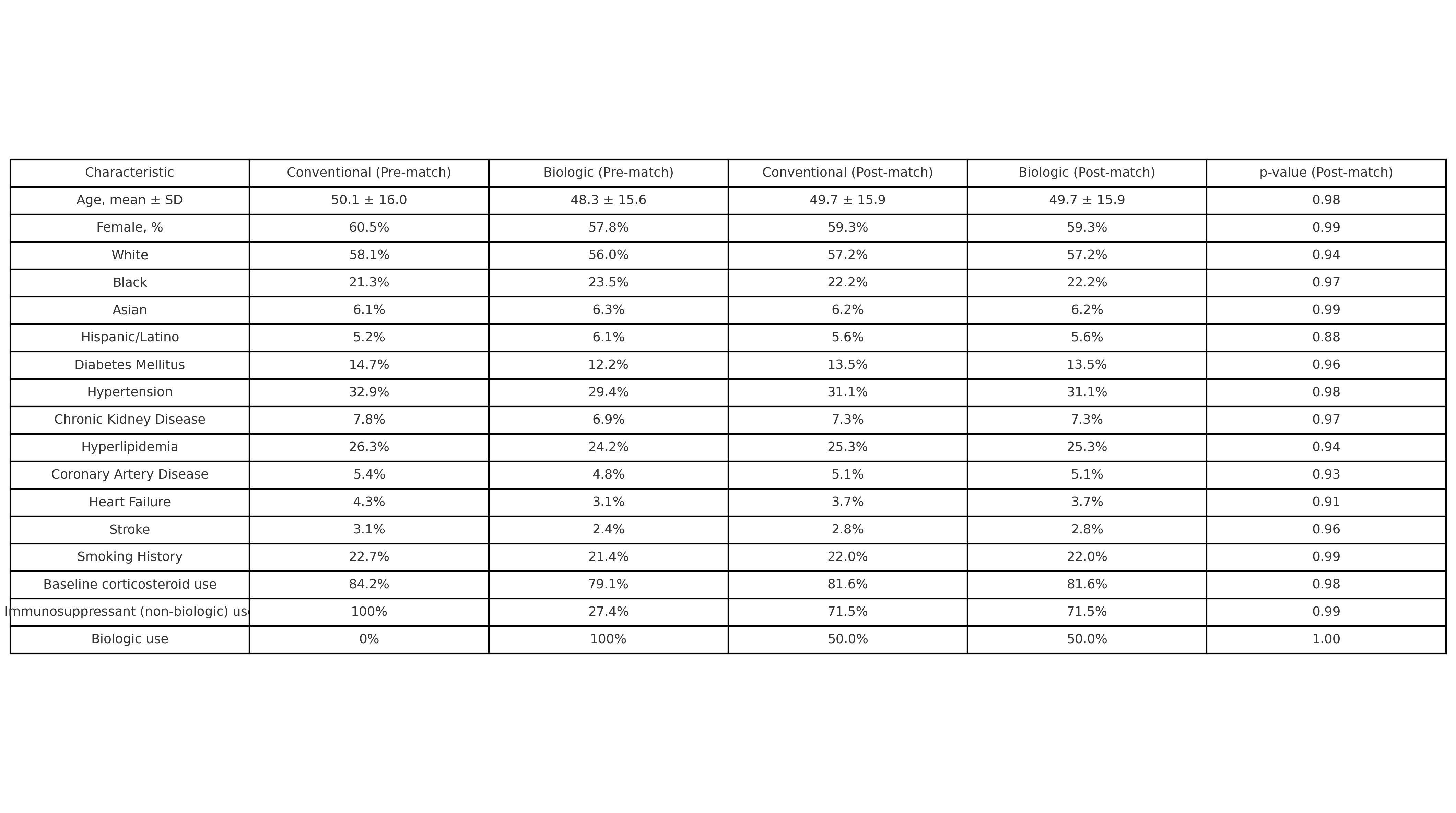
Results: The matched cohort had a mean age of 49.7 ± 15.9 years; 59.3% were female. Racial distribution included White (57.2%), Black (22.2%), Asian (6.2%), and Hispanic/Latino (5.6%).At 1 year, biologics were associated with reduced blindness (0.8% vs. 2.0%; RR 2.57, 95% CI 1.57–4.20; p< 0.001), with similar uveitis flare rates (3.2% vs. 2.5%; p=0.19).At 5 years, biologics significantly reduced glaucoma (4.2% vs. 8.3%; RR 1.96), cataracts (5.3% vs. 8.2%; RR 1.53), macular edema (0.9% vs. 3.0%; RR 3.46), and blindness (2.0% vs. 3.8%; RR 1.85) (p< 0.001 for all). Procedural use was also lower: optical coherence tomography (7.2% vs. 11.0%; RR 1.54) and intravitreal injections (0.9% vs. 3.1%; RR 3.69) (p< 0.001).Systemic complications favored biologics, including sepsis (1.6% vs. 4.1%; RR 2.63), pneumonia (3.5% vs. 5.5%; RR 1.55), heart failure (1.8% vs. 4.3%; RR 2.34), and all-cause mortality (2.4% vs. 5.8%; RR 2.40) (p< 0.001 for all). Cardiovascular events were also reduced: myocardial infarction (1.0% vs. 1.8%; RR 1.84; p=0.008) and major adverse cardiovascular events (MACE) (12.3% vs. 14.6%; RR 1.19; p=0.031).In a matched subgroup analysis (n=317 per group), TNF inhibitors were associated with fewer MACE events than JAK inhibitors (3.1% vs. 9.0%; RR 0.34, 95% CI 0.13–0.86; p=0.017).
Conclusion: Biologic therapy was associated with significantly improved ocular, systemic, cardiovascular, and survival outcomes in patients with rheumatic disease–associated uveitis, without compromising inflammatory control. These findings support earlier use of biologics in this population. Future prospective studies are needed to validate these associations, refine treatment strategies, and identify subgroups most likely to benefit from targeted biologic therapy.
Baseline Demographic, Clinical, and Treatment Characteristics of Patients with Rheumatic Disease–Associated Uveitis Before and After Propensity Score Matching
Comparative Ocular, Procedural, Systemic, and Cardiovascular Outcomes at 1 and 5 Years in Patients with Rheumatic Disease–Associated Uveitis Treated with Biologic versus Conventional Therapy After Propensity Score Matching
Subgroup Comparison of Major Adverse Cardiovascular Events and Other Clinical Outcomes Between TNF and JAK Inhibitors in Biologic-Treated Patients with Rheumatic Disease–Associated Uveitis
To cite this abstract in AMA style:
Akbari H, Abdollahi s. Comparative Effectiveness of Biologic Versus Conventional Therapy in Uveitis with Systemic Rheumatic Diseases: Real-World Evidence from a Propensity-Matched Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparative-effectiveness-of-biologic-versus-conventional-therapy-in-uveitis-with-systemic-rheumatic-diseases-real-world-evidence-from-a-propensity-matched-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-biologic-versus-conventional-therapy-in-uveitis-with-systemic-rheumatic-diseases-real-world-evidence-from-a-propensity-matched-cohort/

.jpg)
.jpg)