Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Avacopan has been approved for adjunctive use in adults with severe active granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA) in the US. Here we describe 12-month effectiveness and safety outcomes for avacopan users in a large healthcare system.
Methods: This ongoing retrospective cohort study involves patients prescribed avacopan for GPA or MPA from 12/1/21-4/1/24. Data from 12 months pre- and post-avacopan prescription are reported. As in the ADVOCATE trial, sustained remission at month 12 is defined as a Birmingham Vasculitis Activity Score version 3 (BVAS v3) of 0 at months 6 and 12 with no glucocorticoid (GC) use for GPA/MPA for 1 month before months 6 and 12, and no relapse from months 6-12. Relapse is assessed after 1 month and is defined as: 1) ≥1 major BVAS v3 item, 2) ≥3 minor items, or 3) 1 or 2 minor items for ≥2 consecutive visits. Increase in BVAS v3 of < 3 and absence of major items defines a minor relapse; all others are defined as a major relapse. We also assessed other disease- and treatment-related outcomes, including the Glucocorticoid Toxicity Index-Metabolic Domains (GTI-MD).
Results: Among 119 patients prescribed avacopan, the mean age was 59.4 years; most were female (83, 70%), newly diagnosed (79, 66%), had GPA (60, 50%), and were MPO-ANCA+ (66, 55%) (Table 1). The median (IQR) baseline BVAS v3 was 12 (8, 17). Most patients received concomitant rituximab (65, 55%), cyclophosphamide (6, 5%), or both (39, 33%). Sixteen (13%) did not initiate avacopan, with the most common reasons being insurance denial (n=5) or high copay (n=3). Of the 103 (87%) initiating avacopan, organ involvement included the kidneys (58, 56%), lungs (50, 49%) and ear, nose, or throat (ENT) (43, 42%) (Table 2).Among 95 with available 12-month follow-up data, 61 (64%) were in remission at month 6, while at month 12, 90 (95%) had a BVAS v3=0, 83 (87%) were GC-free, and 58 (61%) had sustained remission (Table 3). Three patients relapsed (1 major, 2 minor) between months 6 and 12. Overall, 38 (40%) were still on avacopan at 12 months and 57 (60%) discontinued therapy before 12 months mostly due to completion of the provider’s planned treatment course (33/57, 58%) or adverse events (14/57, 25%). The median (IQR) cumulative prednisone-equivalent GC exposure over 12 months was 1050 (280, 2030) mg. Among 84 patients on GCs at baseline, 72 discontinued GCs after a median (IQR) of 84 (56, 175) days, and 12 remained on GCs at 12 months (range of 1.25-20 prednisone-equivalent mg daily). Sustained remission was similar in those with baseline kidney, lung, or ENT involvement.The most common GTI-MD domain with net worsening (i.e., Aggregate Improvement Score [AIS] > 0) was Blood Pressure (41%), followed by Body Mass Index (21%), Lipid Metabolism (18%), & Glucose Tolerance (5%). The majority (53, 56%) had no net worsening of metabolic GC toxicity over 12 months (i.e., AIS ≤0), indicating either net improvement or no change.
Conclusion: In this real-world cohort of individuals with GPA or MPA, among those with 1-year follow-up, most avacopan users discontinued GCs within 3 months and had sustained remission at 12 months, and a minority had avacopan-related adverse events and/or worsening of GC toxicity.
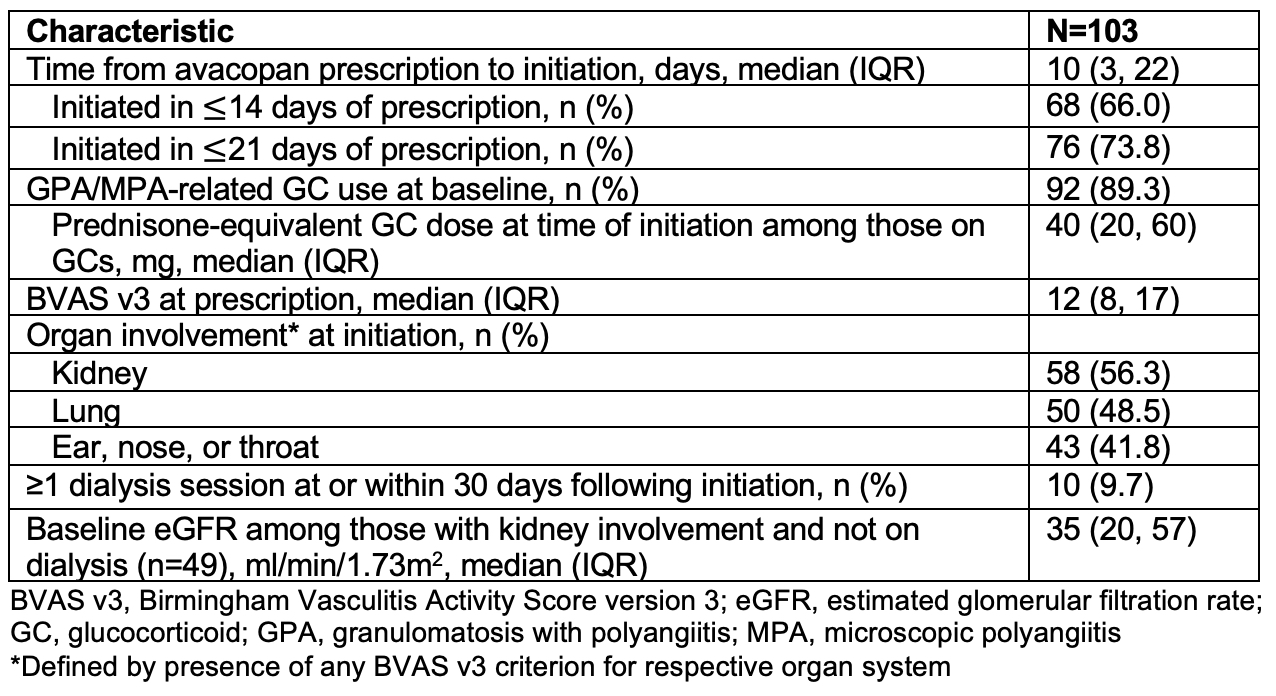 Table 1. Baseline Characteristics of Individuals Prescribed Avacopan for GPA/MPA
Table 1. Baseline Characteristics of Individuals Prescribed Avacopan for GPA/MPA
.jpg) Table 2. Baseline Disease and Treatment-Related Characteristics of Individuals who Initiated Avacopan for GPA/MPA
Table 2. Baseline Disease and Treatment-Related Characteristics of Individuals who Initiated Avacopan for GPA/MPA
.jpg) Table 3. Clinical Outcomes Among Those who Initiated Avacopan for GPA/MPA
Table 3. Clinical Outcomes Among Those who Initiated Avacopan for GPA/MPA
To cite this abstract in AMA style:
Patel N, King A, Negron M, Williams Z, Fernandes A, Shepherd N, Johnson C, Katz G, Arevalo Molina B, Ibiloye E, Oh S, Wallace Z, Unizony S. One Year Real-World Effectiveness with Avacopan in Granulomatosis with Polyangiitis and Microscopic Polyangiitis in a Large Healthcare System [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/one-year-real-world-effectiveness-with-avacopan-in-granulomatosis-with-polyangiitis-and-microscopic-polyangiitis-in-a-large-healthcare-system/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/one-year-real-world-effectiveness-with-avacopan-in-granulomatosis-with-polyangiitis-and-microscopic-polyangiitis-in-a-large-healthcare-system/
