Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The forced vital capacity (FVC) is the most commonly used endpoint in registrational trials for systemic sclerosis-associated interstitial lung disease (SSc-ILD). However, the FVC has known methodological pitfalls, is affected by extra-pulmonary manifestations of SSc and may not be clinically meaningful to patients. Composite endpoints offer promising benefits for SSc-ILD trials in augmenting trial statistical efficiency and including patient-centered components.
Methods: We previously developed a composite endpoint for SSc-ILD using data from the Scleroderma Lung Study (SLS) I, comprised of the FVC, Transitional Dyspnea Index (TDI), Health Assessment Questionnaire Disability Index (HAQ-DI), and the quantitative extent of radiological fibrosis in the zone of maximum fibrosis (QLF-ZM), which demonstrated a more robust treatment effect of cyclophosphamide (CYC) versus placebo than the FVC alone [1]. The purpose of this study was to validate this composite endpoint using data from the SLS II (comparing CYC versus mycophenolate [MMF]). A secondary goal was to determine how the composite endpoint predicted long-term mortality in SLS I and II compared with the change in FVC.
Results: Seventy-two of the 142 randomized participants in SLS II had all of composite endpoint components assessed at 24 months and were included in this analysis. The composite endpoint distributions for SLS I and II were similar (Figure 1). In both SLS I and II, the standardized effect size as determined by Cohen’s d was greater when the composite endpoint model was applied than when the FVC alone model was applied (Table 1). In SLS I and II, the composite endpoint was a better predictor of long-term mortality (HR 0.76 vs 0.98 and 0.59 vs. 0.98, for the composite index vs. FVC based on Cox proportional hazards models in SLS I and II, respectively), although both analyses were likely underpowered to detect statistical significance (Table 2).
Conclusion: The results of this post-hoc analysis provide further evidence that a composite endpoint for SSc-ILD comprised of radiological, physiological and patient-reported outcomes is a promising endpoint for SSc-ILD trials. The composite endpoint may also serve to reduce the sample size required in clinical trials as more patients are needed to detect a significant treatment effect when a single outcome, such as the FVC, is used. References: 1. Volkmann ER, et al. Rheumatology (Sunnyvale). 2015;5:154.
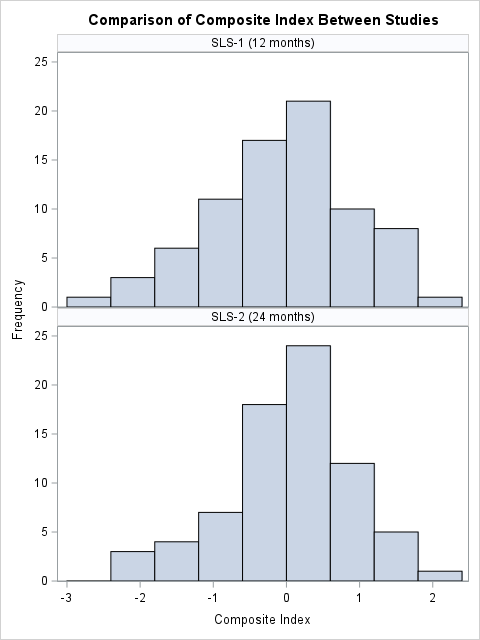 Figure 1. Histogram of composite index distributions in SLS I (top, composite endpoint measured at 12 months) and SLS II (bottom, composite endpoint measured at 24 months). In SLS II, the composite index had a mean (SD) of 0.06 (0.86), indicating slightly reduced variability compared to SLS I (standard normal). This variation may reflect differences in the two cohorts, rates of disease progression, and/or collection timepoints.
Figure 1. Histogram of composite index distributions in SLS I (top, composite endpoint measured at 12 months) and SLS II (bottom, composite endpoint measured at 24 months). In SLS II, the composite index had a mean (SD) of 0.06 (0.86), indicating slightly reduced variability compared to SLS I (standard normal). This variation may reflect differences in the two cohorts, rates of disease progression, and/or collection timepoints.
To cite this abstract in AMA style:
Volkmann E, Wilhalme H, Good S, Kim G, Goldin J, Roth M, Tashkin D. Validation of a Composite Endpoint for Systemic Sclerosis-Associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/validation-of-a-composite-endpoint-for-systemic-sclerosis-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-composite-endpoint-for-systemic-sclerosis-associated-interstitial-lung-disease/

.jpg)
.jpg)