Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
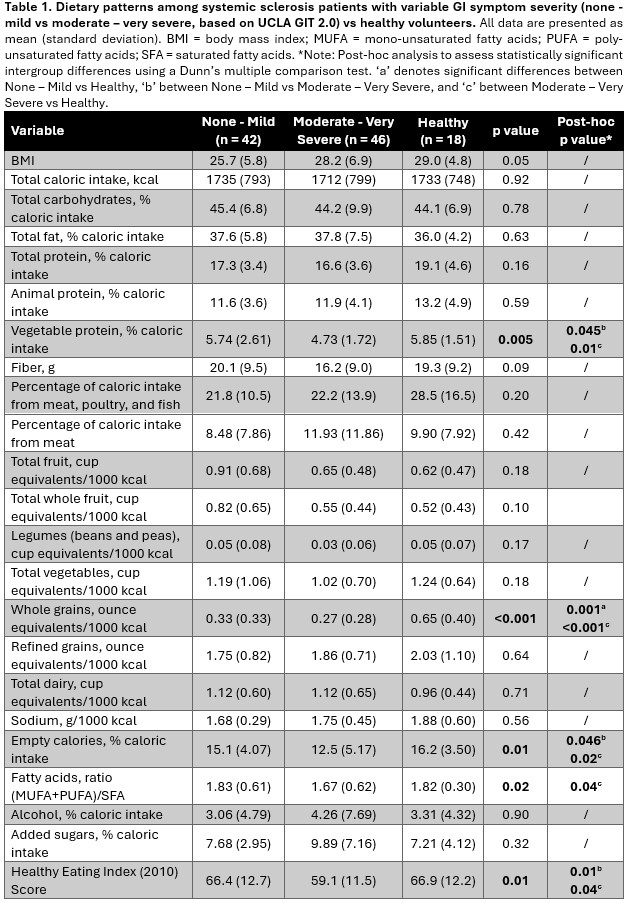
Background/Purpose: Systemic sclerosis (SSc) is a rare multisystem auto-immune disease characterized by peripheral vasculopathy and widespread fibrosis of the skin and internal organs. Up to 90% of SSc patients experience gastrointestinal (GI) symptoms ranging from mild to very severe. We aimed to explore how GI symptom severity correlates with dietary intake patterns and patient-reported outcome measures in SSc patients.
Methods: Adult ( >18 years old) SSc patients meeting the ACR/EULAR 2013 criteria were prospectively recruited and stratified into those with none-to-mild and moderate-to-very severe GI symptoms based on The University of California Los Angeles Scleroderma Clinical Trials Consortium gastrointestinal tract 2.0 (UCLA GIT 2.0) scores.1 Patients completed a food frequency questionnaire to assess dietary intake which was compared to historically obtained healthy volunteer dietary data. Patient-reported outcomes were assessed using a range of validated questionnaires. All variables were compared using Mann-Whitney U tests and Kruskal-Wallis tests and correlated using Spearman’s rank correlation.
Results: 94 SSc participants (79 females; mean 58±12 years) were enrolled. The average UCLA GIT 2.0 score was 0.6±0.4 and participants were categorized as having none to mild (n=47, 50%), and moderate to very severe GI symptoms (n=47, 50%). Compared to healthy volunteers or those with none to mild GI symptoms, patients with moderate to very severe GI symptoms had a lower intake of vegetable proteins and empty calories as well as lower healthy eating index (HEI) scores (Table 1). Patients with moderate to very severe GI symptoms also consumed more saturated fatty acids compared to healthy volunteers. Regardless of GI symptoms, SSc patients consumed fewer whole grains compared to healthy volunteers. Additionally, patients with moderate to very severe GI symptoms reported worse physical and social function, higher levels of fatigue, pain and anxiety, and more bowel movements which were also softer when compared to those with none to mild GI symptoms (Table 2). Harder stool consistency correlated with higher HEI scores (r=0.42, p < 0.001) and a higher intake of fiber (r=0.40, p < 0.001) and vegetable protein (r=0.44, p < 0.001).
Conclusion: In this large prospective single center cohort of well characterized SSc patients, those with moderate to very severe GI symptoms exhibited suboptimal dietary intake and reported worse physical and social functioning as well as higher levels of pain, fatigue and anxiety. Future studies are needed to understand the mechanistic influence of diet in SSc patients and its impact on GI symptoms and other patient-reported outcomes.
To cite this abstract in AMA style:
Guedens T, Lesmana E, Edwinson A, Breen-Lyles M, Karn A, Grover M, Makol A. Gastrointestinal Symptom Severity Is Associated With Worse Patient-Reported Outcomes And Dietary Patterns In Systemic Sclerosis: A Single Center Prospective Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/gastrointestinal-symptom-severity-is-associated-with-worse-patient-reported-outcomes-and-dietary-patterns-in-systemic-sclerosis-a-single-center-prospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gastrointestinal-symptom-severity-is-associated-with-worse-patient-reported-outcomes-and-dietary-patterns-in-systemic-sclerosis-a-single-center-prospective-study/

.jpg)