Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with systemic lupus erythematosus (SLE) can develop cardiovascular (CV) damage (angina, myocardial infarction, ventricular dysfunction, valvular disease, or pericarditis for 6 months/pericardiectomy) particularly in patients with uncontrolled disease activity, flares, or under long-term glucocorticoid use. The Treatment of Uncontrolled Lupus via the Interferon Pathway (TULIP) trials and long-term extension (LTE) study demonstrated that anifrolumab can control disease activity and reduce the need for glucocorticoid use. However, evaluation of the long-term effects of anifrolumab on CV damage remains limited by patient attrition in the placebo arm and clinical trial duration. The purpose of this analysis—the Long-Term Organ Damage: Anifrolumab versus Real-World Standard of Care in Adult Patients with Active SLE (LASER; NCT06485674)—was to (1) assess the mean CV damage accrual at 4 years, and (2) estimate time to first CV event up to 4 years of therapy in adult patients with moderately to severely active SLE treated with anifrolumab or real-world standard of care (RW SOC).
Methods: This analysis used an external control arm of patients who received RW SOC from the University of Toronto Lupus Clinic (UTLC). Patients assigned to anifrolumab 300 mg in the TULIP-1 and -2 trials were included in the anifrolumab arm (n = 354). Patients from the UTLC registry (n = 561) with matching eligibility criteria comprised the RW SOC arm. Baseline confounding and possible informative loss to follow-up were accounted for using propensity scores (PS) and inverse probability censoring weights, respectively. Fourteen known predictors of CV damage were included in the PS models, and predictors that appeared imbalanced after PS weighting (e.g., standardized mean difference ≥ 0.2) were included as independent covariates in the outcome model. Mean changes in CV-related Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) from baseline to year 4 were estimated using weighted linear regression models in the average treatment effect in the control (ATC), treated (ATT), and overlap (ATO) groups. Time to first CV event up to 4 years was assessed using Kaplan–Meier estimators and a Cox proportional hazards regression model.
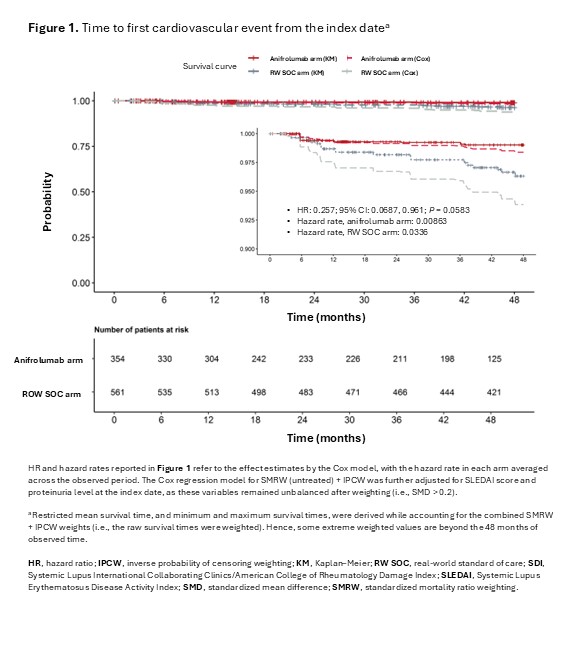
Results: Baseline patient characteristics were adequately balanced after PS weighting. Patients in the anifrolumab arm accrued (on average) 0.046 fewer CV SDI points (95% confidence interval [CI]: −0.074, −0.018; P = 0.008) at 4 years compared with patients in the RW SOC arm (Table 1). Results were consistent across ATC and ATT groups. A total of 6 patients in the anifrolumab arm vs 17 patients in the RW SOC arm experienced CV events (Table 2). Patients in the anifrolumab arm were 74.3% less likely to experience a CV event over 4 years compared to patients in the RW SOC arm (hazard ratio, 0.257; 95% CI: 0.0687, 0.961; P = 0.0583; Figure 1).
Conclusion: Patients who received anifrolumab in the TULIP trials and LTE accumulated less CV damage at 4 years compared with patients who received RW SOC in an external control arm. These results support the added benefit of anifrolumab to RW SOC in preventing long-term CV organ damage in patients with moderately to severely active SLE.
To cite this abstract in AMA style:
Touma Z, Bruce I, Furie R, Morand E, Tummala R, Chandran S, Abreu G, Knagenhjelm J, Emmas C, Agustin L, Venerus A, Mehdikhanova T, Yang Z, Waratani M. Treatment With Anifrolumab Prevents Long-Term Cardiovascular Damage Accrual Compared With Real-World Standard of Care in Patients With Systemic Lupus Erythematosus: Findings From the LASER Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/treatment-with-anifrolumab-prevents-long-term-cardiovascular-damage-accrual-compared-with-real-world-standard-of-care-in-patients-with-systemic-lupus-erythematosus-findings-from-the-laser-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-with-anifrolumab-prevents-long-term-cardiovascular-damage-accrual-compared-with-real-world-standard-of-care-in-patients-with-systemic-lupus-erythematosus-findings-from-the-laser-study/

.jpg)
.jpg)