Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus Nephritis (LN) is a serious complication of systemic lupus erythematosus (SLE), occurring in up to 50% of patients. Currently, Anifrolumab (ANI) is approved for moderate-to-severe SLE, based on clinical trials excluding active LN (1,2). In LN, an elevated type I IFN gene signature (IFNGS) is associated with increased disease activity, high proteinuria levels, and therapeutic failure (3). In patients with LN treated with ANI in clinical practice in Spain, our aim was to assess the effectiveness and safety of ANI.
Methods: Observational multicenter study of patients diagnosed with SLE (EULAR/ACR 2019 classification criteria), treated with ANI who presented renal involvement (renal insufficiency and/or hematuria and/or proteinuria). Data were collected from medical records up to January 31 2025. Demographic, clinical, laboratory, and pathologic variables were evaluated, along with previous and concomitant therapies, disease activity indices (SLE-DAS, SLEDAI-2K, PGA), organ damage index (SLICC SDI), and safety.
Results: We studied 18 patients (16 women/2 men), mean age of 38.16±10.35 years (range 20-63 years), from 14 hospitals. Baseline characteristics, prior treatment before starting ANI and LN subtypes are summarized in TABLE. The most common renal manifestations at ANI initiation were proteinuria (n=9, 50%), hematuria (n=5; 27.8%), and renal insufficiency (n=2; 11.1%). The mean number of immunosuppressive treatments (synthetic/biologic) received prior to ANI was 4.3 ± 2.4 (range 1-12). All of them had received belimumab, and 15 mycophenolate mofetil. The standard ANI regimen was 300 mg every 4 weeks, except in two patients who received loading doses (900 mg/4 weeks for 3 months, then 300 mg/4 weeks).In addition to corticosteroids, ANI was administered concomitantly with antimalarials (n=16), mycophenolate mofetil (n=11), tacrolimus (n=4), azathioprine (n=2), methotrexate (n=1), and voclosporin (n=1). A rapid (from the first month) and maintained significant improvement was observed in: a) disease activity (SLE-DAS, SLEDAI-2K, PGA) (Figure 1) b) immunologic markers (decrease in anti-dsDNA antibody titers and normalization of C3 and C4 levels) c) renal parameters (reduction in creatinine, proteinuria and improvement in glomerular filtration rate) (Figure 2). After a mean follow-up of 8.8 ± 5.5 months, a reduction in the number of relapses was observed, from a median [IQR] of 2 [0-3] to 0 [0-0]. The organ damage index remained stable. All 18 patients remained on ANI, and the most relevant adverse events were herpes zoster (n=1) and hidradenitis suppurativa (n=1). The prednisone dose was decreased from 9.2±7.9 mg/day to 3.3±2 mg/day (p= 0.1).
Conclusion: To our knowledge, this is the first real-life study of ANI in LN. In refractory patients to multiple immunosuppressive therapies, we observed a rapid and maintained effectiveness in SLE activity and renal manifestations, with a good safety profile. These preliminary data should be confirmed in clinical trials.
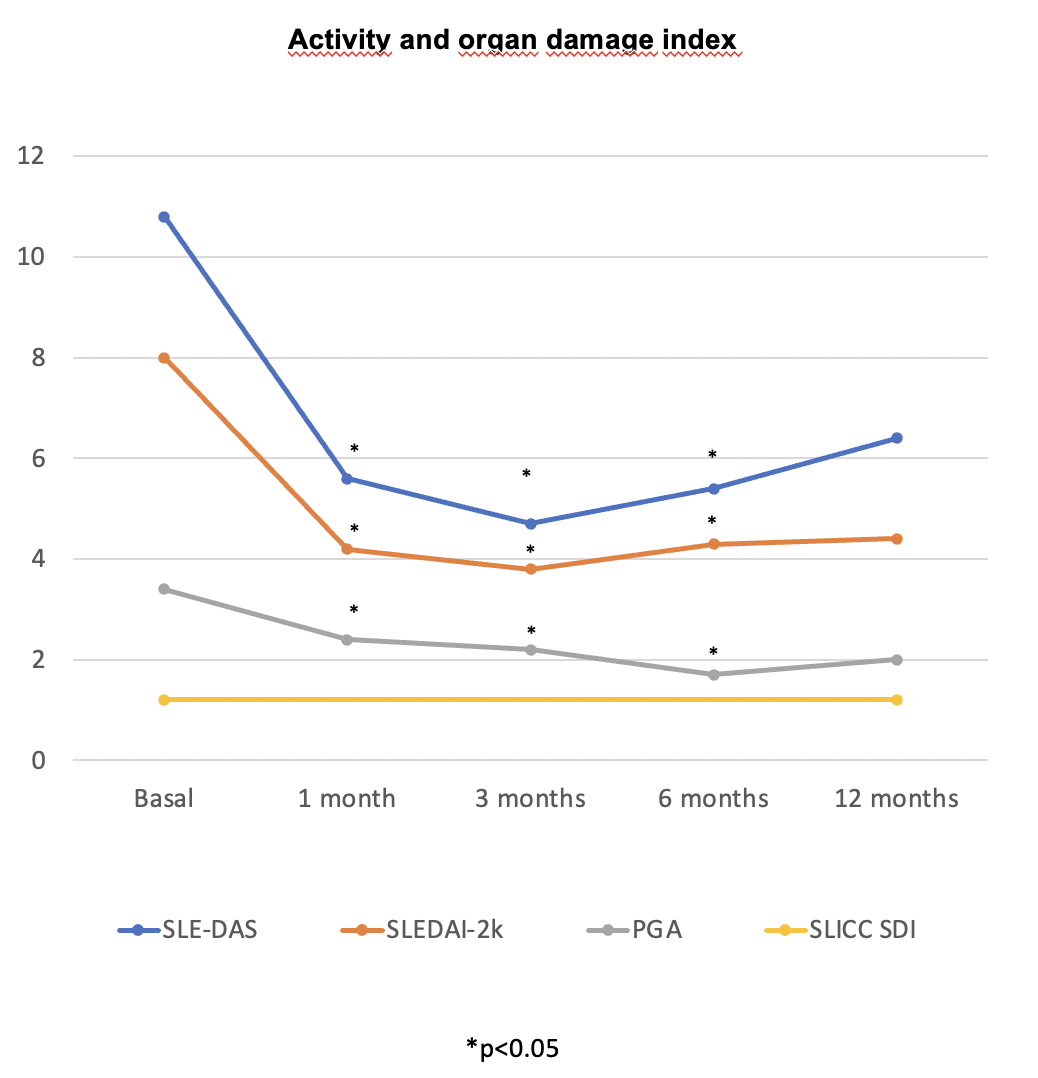 Table. Clinical manifestations at the time of anifrolumab initiation and previously treatments.
Table. Clinical manifestations at the time of anifrolumab initiation and previously treatments.
Abbreviations in alphabetical order: ANI: anifrolumab; AZA: azathioprine; BLM: belimumab; CYM: cyclophosp-hamide; MMF: mycophenolate mofetil; MP: methylprednisolone; MTX: methotrexate; LN: lupus nephritis; RTX: rituximab
.jpg) Figure 1. Evolution of activity and organ damage index after the initiation of anifrolumab.
Figure 1. Evolution of activity and organ damage index after the initiation of anifrolumab.
.jpg) Figure 2. Evolution of creatinine levels, glomerular filtration rate and proteinuria (g/24h) after the initiation of anifrolumab.
Figure 2. Evolution of creatinine levels, glomerular filtration rate and proteinuria (g/24h) after the initiation of anifrolumab.
To cite this abstract in AMA style:
Calvo-Río V, Secada-Gómez C, Garcia-Magallon B, Palma-Sanchez D, García-Valle A, Urionaguena Onaindia I, Mayo-Juanatey A, Hernández Velasco P, GONZALEZ ARRIBAS G, Pareja-Martínez A, Corteguera M, Sada Urmeneta G, Collado Ramos P, Tandaipan J, Miguelez Sanchez J, Blanco R. Anifrolumab In Systemic Lupus Erythematosus With Renal Involvement: National Multicenter Registry In Clinical Practice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anifrolumab-in-systemic-lupus-erythematosus-with-renal-involvement-national-multicenter-registry-in-clinical-practice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anifrolumab-in-systemic-lupus-erythematosus-with-renal-involvement-national-multicenter-registry-in-clinical-practice/
