Session Information
Date: Monday, October 27, 2025
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: About 70% of patients with primary Sjögren’s disease (pSjD) suffer from fatigue. Fatigue is associated with functional deficits(1), leading to anxiety, depression and reduced quality of life. Recent studies showed that sleep apnea syndrome (SAS) is common in patients with pSjD(2,3).We aimed to determine the prevalence of SAS in pSjD patients and to examine to what extent SAS treatment could improve fatigue and disease activity in pSjD.
Methods: We report the 4-week (W4), 3-month (V2) and 6-month (V3) data of patients included between 2019-2024. Three different cohorts (pSjD, psoriatic arthritis (PsA) and patients with SAS without underlying rheumatologic disease (SAS+)) were screened for SAS and compared. All patients with pSjD and PsA fulfilled the respective ACR/EULAR criteria for their diseases. An apnoea-hypopnoea index >5/h triggered treatment with CPAP or positional therapy. Assessments after W4 comprised fatigue (FACIT), visual analogue scale for pain and quality of life, depression (PHQ-9), quality of life (WHOQOL-BREF), daytime sleepiness (EPS) and ESSPRI. Assessments at inclusion (V1), V2 and V3: CRP and ESSDAI. Level of significance was set at a p-value < 0.05.
Results: The Departments of Rheumatology and Clinical Immunology, University Medical Center in Freiburg and Strasbourg examined 41 patients with pSjD (age 54.98 ± 12.36) and 21 patients with PsA (age 56.00 ± 9.12) for SAS. The SAS+ control group (age 58.46 ± 9.90) comprised of 13 individuals. The pSjD+ patients were significantly older than the pSjD- patients (p=0.00076). In pSjD, 15/41 (36.6%) of patients were diagnosed with SAS; 13/15 were treated with CPAP or positional therapy (pSjD+). In PsA, 11/ 21 patients had SAS; 8/11 received CPAP or positional therapy (PsA+). In SAS, 13/13 received CPAP treatment. Between V1 and V2, and V1 and V3, the FACIT score improved significantly in SAS+ and PsA+ patients (p=0.008, p=0.012; p=0.017, p=0.034). Furthermore, in patients with pSjD+, the ESSDAI and ESSPRI (improved domains: fatigue and limb pain) scores improved significantly between V1 and V3 (p=0.003; p=0.043), while the Soc WHOQOL-BREF score significantly decreased between V1 and V2 (p=0.017). In PsA+ patients the PHQ-9 score improved significantly between V1 and V2 (p=0.020), and V1 and V3 (p=0.035), the Health WHOQOL-BREF and the Env WHOQOL-BREF scores improved significantly between V1 and V2 (p=0.042; p=0.044), the EPS between V1 and V3 (p=0.014). Table 1 gives further details.Interestingly in PsA- patients, the following significant improvements were noted: joint tenderness between V1 and V2 (p=0.036), PHQ-9 between V1 and W4 (p=0.017), and FACIT between V1 and V2 (p=0.036).
Conclusion: SAS affected 36.6% of pSjD patients, which is within the range of analyses published in literature. In individuals with pSjD, adequate SAS treatment markedly reduced disease activity and symptoms; this implies that SAS screening in pSjD is in general appropriate.Successful immunosuppressive treatment might positively influence PHQ-9 and FACIT in PsA- patients.
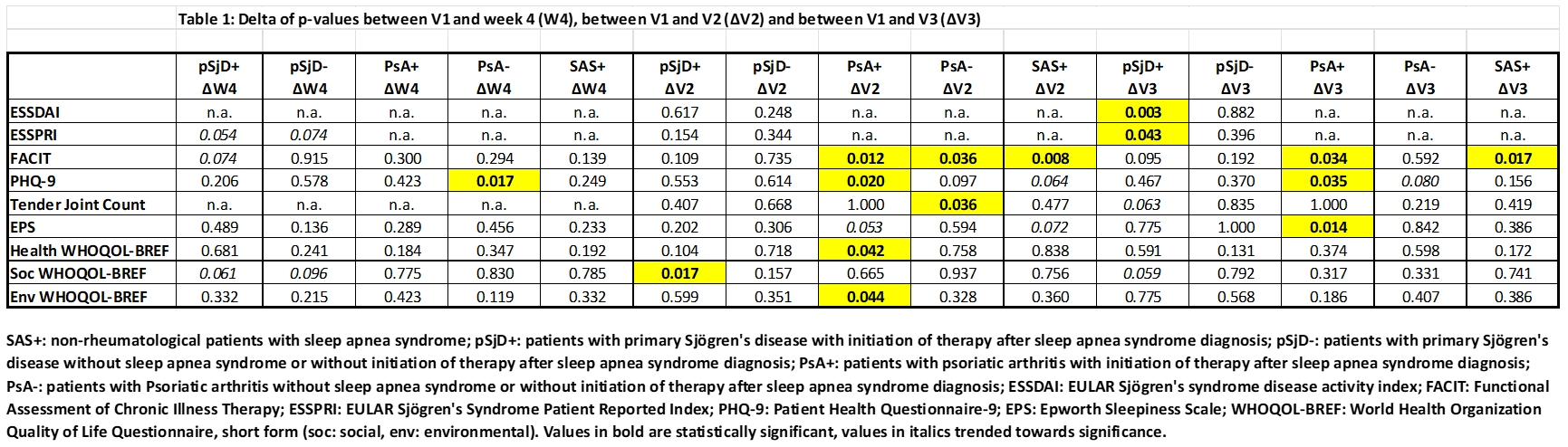 SAS+: non-rheumatological patients with sleep apnea syndrome; pSjD+: patients with primary Sjögren’s disease with initiation of therapy after sleep apnea syndrome diagnosis; pSjD-: patients with primary Sjögren’s disease without sleep apnea syndrome or without initiation of therapy after sleep apnea syndrome diagnosis; PsA+: patients with psoriatic arthritis with initiation of therapy after sleep apnea syndrome diagnosis; PsA-: patients with Psoriatic arthritis without sleep apnea syndrome or without initiation of therapy after sleep apnea syndrome diagnosis; ESSDAI: EULAR Sjögren’s syndrome disease activity index; FACIT: Functional Assessment of Chronic Illness Therapy; ESSPRI: EULAR Sjögren’s Syndrome Patient Reported Index; PHQ-9: Patient Health Questionnaire-9; EPS: Epworth Sleepiness Scale; WHOQOL-BREF: World Health Organization Quality of Life Questionnaire, short form (soc: social, env: environmental). Values in bold are statistically significant, values in italics trended towards significance.
SAS+: non-rheumatological patients with sleep apnea syndrome; pSjD+: patients with primary Sjögren’s disease with initiation of therapy after sleep apnea syndrome diagnosis; pSjD-: patients with primary Sjögren’s disease without sleep apnea syndrome or without initiation of therapy after sleep apnea syndrome diagnosis; PsA+: patients with psoriatic arthritis with initiation of therapy after sleep apnea syndrome diagnosis; PsA-: patients with Psoriatic arthritis without sleep apnea syndrome or without initiation of therapy after sleep apnea syndrome diagnosis; ESSDAI: EULAR Sjögren’s syndrome disease activity index; FACIT: Functional Assessment of Chronic Illness Therapy; ESSPRI: EULAR Sjögren’s Syndrome Patient Reported Index; PHQ-9: Patient Health Questionnaire-9; EPS: Epworth Sleepiness Scale; WHOQOL-BREF: World Health Organization Quality of Life Questionnaire, short form (soc: social, env: environmental). Values in bold are statistically significant, values in italics trended towards significance.
To cite this abstract in AMA style:
Kuhn A, Seng M, Frye B, Fähndrich S, Vollmer L, GOTTENBERG J, Kollert F, Voll R, Finzel S. Treatment of sleep apnoea syndrome in patients with primary Sjögren’s disease improves symptoms and activity of disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/treatment-of-sleep-apnoea-syndrome-in-patients-with-primary-sjogrens-disease-improves-symptoms-and-activity-of-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-sleep-apnoea-syndrome-in-patients-with-primary-sjogrens-disease-improves-symptoms-and-activity-of-disease/
