Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: According to the 2022 EULAR recommendations for the management of rheumatoid arthritis (RA), the presence of rheumatoid factor (RF), particularly at high levels, is recognised as a poor prognostic factor.1 In patients with poor prognosis who fail to achieve treatment targets with initial conventional synthetic DMARD (csDMARD) therapy, the addition of a biological DMARD (bDMARD) is recommended.2,3 Despite this recommendation, patients with high RF levels are associated with reduced response to monoclonal anti-TNF therapies.4 Certolizumab pegol (CZP), a TNF inhibitor without a fragment crystallizable (Fc) region, may offer improved efficacy for these patients.5,6 Given the unmet need in patients with RA and high RF levels, who are less likely to respond to Fc-containing TNF inhibitors and are considered at poor prognostic risk, we evaluated the cost per responder (CPR) for CZP versus adalimumab (ADA) to support value-based treatment decisions from the perspective of Canada and Brazil over a 104 week time horizon.
Methods: A 104-week (Wk) CPR model was developed using data from EXXELERATE,7 a phase IV, randomised, head-to-head trial comparing CZP plus methotrexate (MTX) with ADA plus MTX, in patients with moderate to severe RA. Response rates were defined by achievement of low disease activity (LDA), measured as a Disease Activity Score 28 joints-C-reactive protein (DAS28-CRP) ≤2.7. CPR at Wk104 was calculated by multiplying the total number of doses administered over 104 weeks by the drug acquisition cost divided by the corresponding response rate. Treatment regimens followed the approved product labels: CZP 400 mg at Weeks 0, 2, and 4, followed by 200 mg every 2 weeks; ADA 40 mg every other week. Average prices were considered to derive ADA biosimilar costs.
Results: At Wk104, the LDA achievement rates were higher with CZP compared to ADA (42.5% vs 25.5%). The cost per LDA achievement for CZP vs ADA respectively, was CAD 94,348 vs CAD 97,828 in Canada, and BRL 156,203 vs BRL 793,888 in Brazil (Table 1 and 2).A sensitivity analysis was performed to test the robustness of these findings under varying list-to-net pricing assumptions. Across multiple scenarios, CZP consistently maintained a more favourable cost profile compared to ADA, highlighting its potential value under a range of pricing conditions.
Conclusion: At Wk104, CZP at list price demonstrated a lower CPR compared to ADA biosimilar price in both Canada and Brazil. These findings suggest that, for patients with moderate to severe active rheumatoid arthritis and high rheumatoid factor levels, CZP may offer a more personalised and cost-effective treatment strategy, supporting more efficient allocation of healthcare resources and potentially reducing the budgetary burden for payers in Canadian and Brazilian healthcare settings.References: 1. Smolen JS. Ann Rheum Dis. 2023;82(1):3–18; 2. Cuchacovich M. Clin Rheumatol 2014;33(12):1707–14; 3. Takeuchi T. Arthritis Res 2017;19:194, 4. Pappas DA. Rheumatol Int 2021;41:585–93; 5. Bidgood SR. ACR 2024; 6. Smolen JS. Rheumatology. 2024;63(11):3015–24; 7. Smolen JS. Lancet 2016; 388(10061):2763-2774.
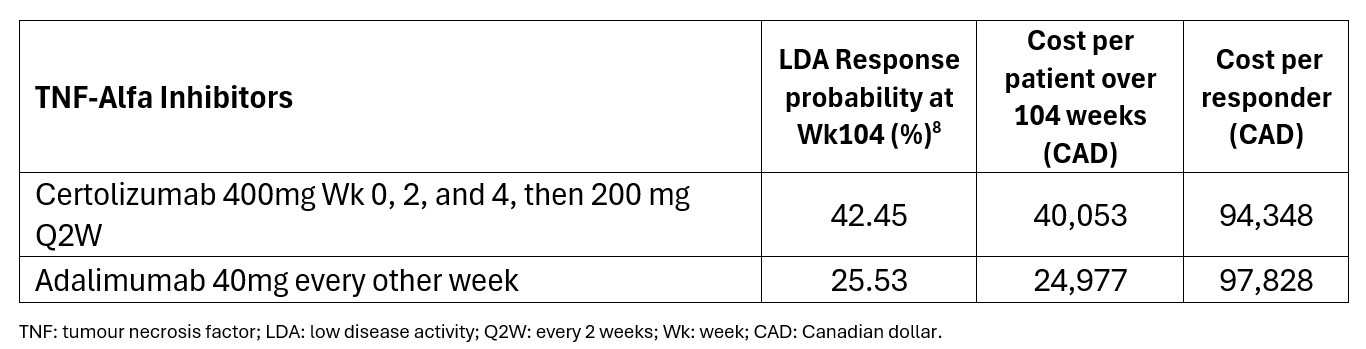 Table 1: Canada – Cost Per Responder in Local Currency
Table 1: Canada – Cost Per Responder in Local Currency
.jpg) Table 2: Brazil – Cost Per Responder in Local Currency
Table 2: Brazil – Cost Per Responder in Local Currency
To cite this abstract in AMA style:
Marty R, Ufuktepe B, Tilt N, Decuypere F, Blake A, Kiri S, Soomro M. Cost Per Responder Analysis of Certolizumab Pegol Versus Adalimumab in Patients with Moderate to Severe Active Rheumatoid Arthritis and High Rheumatoid Factor Levels: A Payer Perspective from Canada and Brazil [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/cost-per-responder-analysis-of-certolizumab-pegol-versus-adalimumab-in-patients-with-moderate-to-severe-active-rheumatoid-arthritis-and-high-rheumatoid-factor-levels-a-payer-perspective-from-canada-a/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-per-responder-analysis-of-certolizumab-pegol-versus-adalimumab-in-patients-with-moderate-to-severe-active-rheumatoid-arthritis-and-high-rheumatoid-factor-levels-a-payer-perspective-from-canada-a/
