Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Methotrexate (MTX) and leflunomide (LEF) are commonly used disease-modifying antirheumatic drugs (DMARDs) in the treatment of rheumatoid arthritis (RA). While both are widely prescribed, their comparative efficacy and safety remain a subject of clinical debate. This systematic review and meta-analysis aimed to evaluate and compare the efficacy and safety outcomes of MTX and LEF monotherapies in RA patients.
Methods: We conducted a systematic review and Meta-analysis using PRISMA guidelines. A comprehensive literature search was conducted in Medline, Embase, and the Cochrane Library from inception to 2025 to identify clinical trials and observational studies comparing MTX and LEF. A total of 7920 articles were initially identified. After removing duplicates and screening titles and abstracts, 39 full-text articles were reviewed, and 10 studies met the inclusion criteria. The quality analysis was conducted by two independent reviewers using the Cochrane RoB 2 for RCTs and the Newcastle Ottawa tool for observational studies. Primary outcomes were comparative efficacy and safety of both drugs. A random-effects model was used to perform meta-analyses, with odds ratios (ORs) calculated for efficacy outcomes and risk ratios (RRs) for safety outcomes to compare the two treatment groups. Heterogeneity among studies may affect the comparability of outcomes.
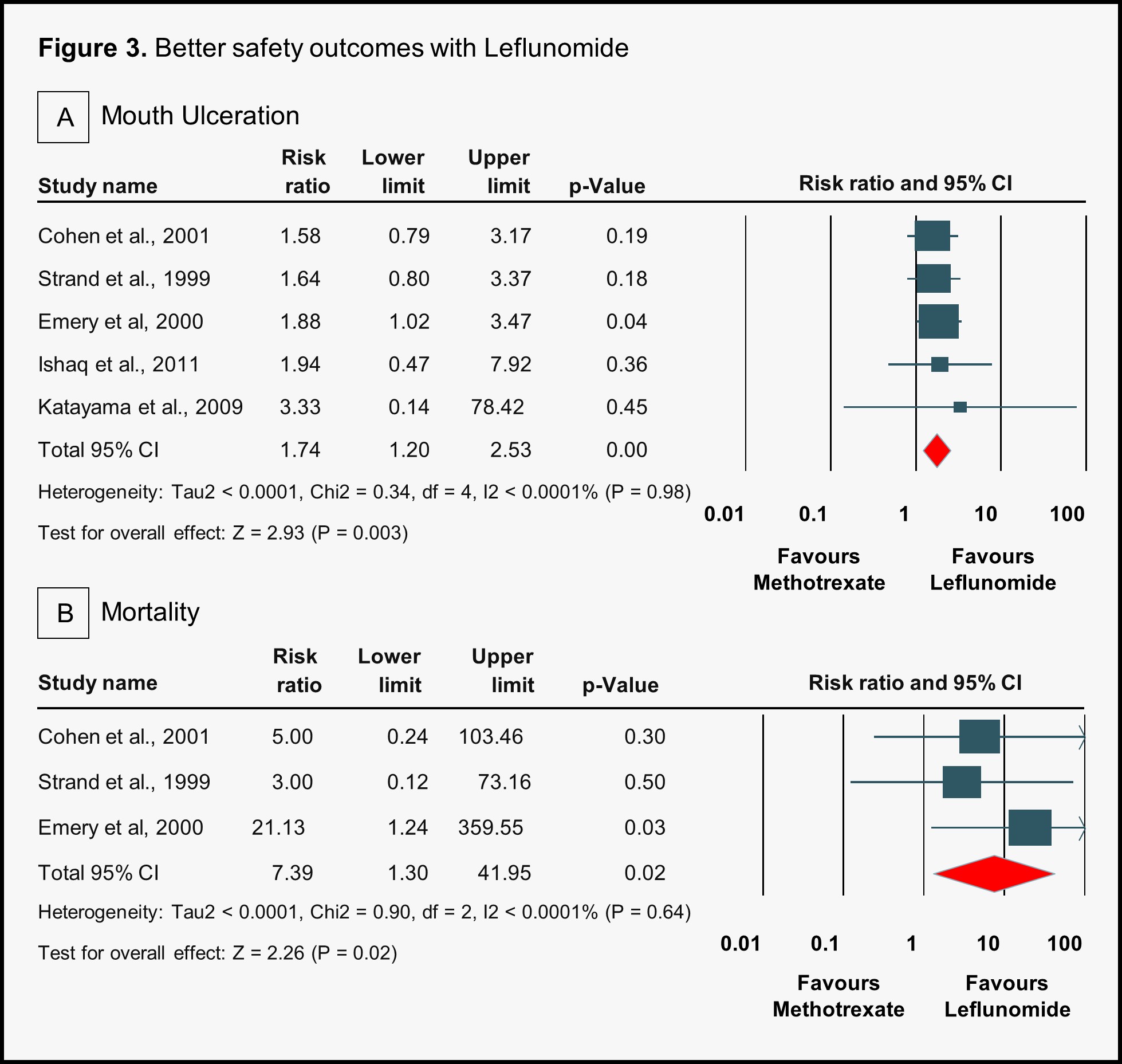
Results: Ten studies were included for comparative analysis, comprising 6 RCTs and 4 observational studies, with a total of 5514 participants (2059 in the LEF group and 3455 in the MTX group). The study population had a mean age of approximately 51.5 ± 0.21 years and a female predominance of 78.39%. Efficacy analysis revealed that methotrexate was significantly less effective than leflunomide in achieving a 70% improvement in ACR response (ACR ≥70) at 12 months (OR 0.46, 95% CI 0.22–0.94, p = 0.03) (Figure 1). No other ACR response comparisons at 12 or 24 months showed statistically significant differences between the two treatments. Regarding safety outcomes, methotrexate was associated with a lower risk of diarrhea (RR 0.50, 95% CI 0.40–0.63, p < 0.001), alopecia (RR 0.61, 95% CI 0.47–0.80, p = 0.0004), withdrawal due to adverse events (RR 0.71, 95% CI 0.59–0.85, p = 0.0002), and hypertension (RR 0.34, 95% CI 0.23–0.52, p < 0.001) compared to leflunomide (Figure 2). However, it was associated with a higher risk of mouth ulceration (RR 1.74, 95% CI 1.20–2.53, p = 0.003) and mortality (RR 7.39, 95% CI 1.30–41.95, p = 0.02) (Figure 3).
Conclusion: This meta-analysis demonstrates significant differences in both efficacy and safety outcomes between the two treatment groups. Leflunomide showed superior efficacy compared to methotrexate in achieving a high-level ACR response at 12 months, while methotrexate was associated with a more favorable safety profile, including lower risks of diarrhea, alopecia, hypertension, and treatment withdrawal.
 Comparison of the statistically significant efficacy of Methotrexate and Leflunomide
Comparison of the statistically significant efficacy of Methotrexate and Leflunomide
.jpg) Comparison of safety outcomes: Methotrexate and Leflunomide with Methotrexate showing superior outcomes
Comparison of safety outcomes: Methotrexate and Leflunomide with Methotrexate showing superior outcomes
.jpg) Comparison of safety outcomes: Methotrexate and Leflunomide, with Leflunomide showing superior outcomes
Comparison of safety outcomes: Methotrexate and Leflunomide, with Leflunomide showing superior outcomes
To cite this abstract in AMA style:
Pitliya A, Vasudevan S, Sharma S, Jeswani B, Ratnani T, Thirumaran R. The Better Disease-modifying antirheumatic drug (DMARD)? Efficacy and Safety of Methotrexate Versus Leflunomide Monotherapy in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-better-disease-modifying-antirheumatic-drug-dmard-efficacy-and-safety-of-methotrexate-versus-leflunomide-monotherapy-in-rheumatoid-arthritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-better-disease-modifying-antirheumatic-drug-dmard-efficacy-and-safety-of-methotrexate-versus-leflunomide-monotherapy-in-rheumatoid-arthritis-a-systematic-review-and-meta-analysis/
