Session Information
Date: Monday, October 27, 2025
Title: (1306–1346) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The increasing use of glucagon-like peptide-1 receptor agonists (GLP1-RAs) for diabetes mellitus (DM) and weight loss has the potential modify the risk for rheumatoid arthritis (RA) development. In this study we aimed to explore the association between GLP1-RA use and RA development.
Methods: This case-control study utilized the Leumit Health Services (LHS) computerized database. Cases were defined as adult RA patients (≥18 years) registered in the database up to December 31, 2023, using ICD-9 code 714.0, who also were prescribed conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs) or diagnosed with RA by a rheumatologist. Each case was matched with five controls based on age, gender, and socioeconomic status from the same database. The primary exposure was the use of either liraglutide, dulaglutide, or semaglutide within the 5 years preceding the RA diagnosis (index date). Demographic variables, smoking status, comorbidities, and malignancy history were collected at the index date. Univariate and multivariable logistic regression analyses were performed to evaluate the association between GLP1-RA usage and RA, with the multivariable model adjusting for potential confounders. A p-value of less than 0.05 was considered statistically significant. Ethical approval for the study was obtained from the LHS Institutional Review Board.
Results: The study included 4,405 RA patients and 22,025 matched controls. Baseline characteristics revealed that RA patients had a significantly higher BMI and a greater prevalence of several comorbidities including DM, chronic obstructive pulmonary disease, osteoporosis, hypothyroidism, inflammatory bowel disease, and fibromyalgia (Table 1). GLP1-RA use was non-significantly associated with RA: liraglutide (OR 1.38, p=0.051), dulaglutide (OR 1.54, p=0.101), and semaglutide (OR 1.38, p=0.075) (Table 2). After adjusting for age, smoking status, and DM in a multivariate model, these associations remained non-significant (Table 3). Notably, DM was identified as an independent risk factor for RA (adjusted OR 1.18, p=0.001) (Table 3).
Conclusion: Our findings demonstrated that GLP1-RAs exposure was not associated with a decreased incidence of RA, but rather the exposure trended towards increased incidence. DM was the only variable that was significantly associated with incident RA, pointing to the potential role of metabolic dysregulation in the development of autoimmune disease. Further prospective, interventional studies are warranted to understand the effect of GLP1-RA on the risk of developing RA.
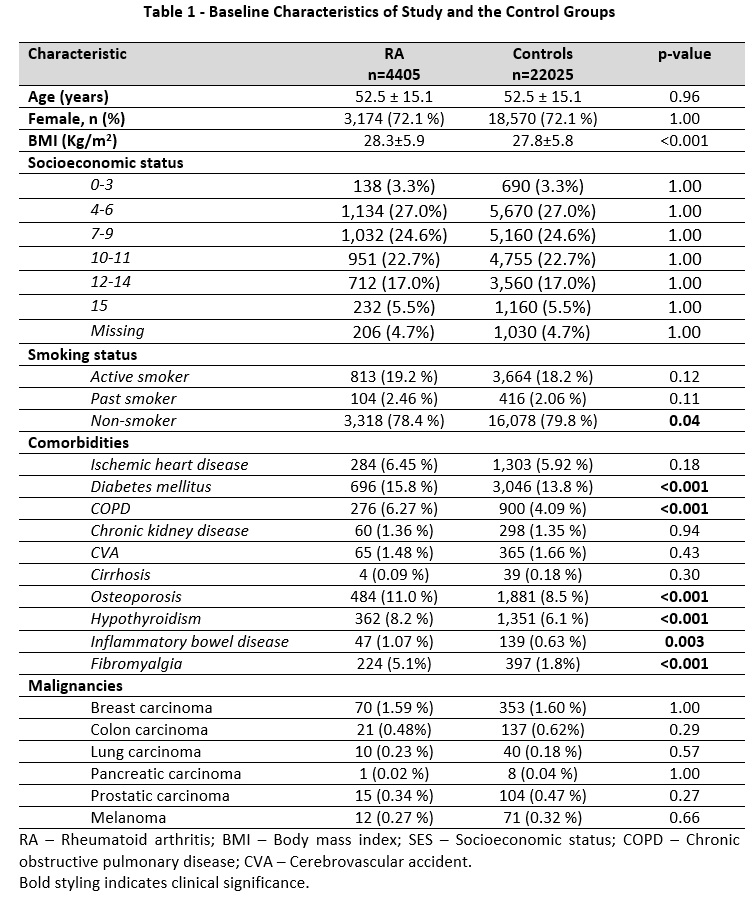 Table 1 – Baseline Characteristics of Study and the Control Groups
Table 1 – Baseline Characteristics of Study and the Control Groups
.jpg) Table 2 – Univariate Analysis of the Associations Between GLP1-RA Use and RA
Table 2 – Univariate Analysis of the Associations Between GLP1-RA Use and RA
.jpg) Table 3 –Adjusted Multivariate Analysis for Factors Associated with RA
Table 3 –Adjusted Multivariate Analysis for Factors Associated with RA
To cite this abstract in AMA style:
Israel A, Hassan F, Kurtam J, Awad J, Merzon E, Naffaa M. The Association Between Glucagon-Like Peptide-1 Receptor Agonist and Rheumatoid Arthritis: A Population-Based Case Control Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-association-between-glucagon-like-peptide-1-receptor-agonist-and-rheumatoid-arthritis-a-population-based-case-control-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-between-glucagon-like-peptide-1-receptor-agonist-and-rheumatoid-arthritis-a-population-based-case-control-study/
