Session Information
Date: Monday, October 27, 2025
Title: (1306–1346) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: rheumatoid arthritis (RA) is characterized by a lipid paradox induced by systemic inflammation. JAK inhibitors (JAKi) have been associated with a short-term increase in total cholesterol (TC), LDL and HDL fractions, which could be a direct effect of the drug, or indirectly related to drug-induced systemic inflammation reduction. The aim of this study was to investigate the course of TC levels and fractions following the introduction of a JAKi.
Methods: Retrospective study conducted at a single center, encompassing all RA patients who initiated JAKi therapy between 2019 and 2023. The study population included patients who had at least two follow-up visits and had a lipid panel available at the time of enrollment. Patients who had received statin therapy at the time of enrollment were excluded.The primary endpoint was defined as changes in TC and fractions at 6 months from the initiation of JAKi treatment. Secondary endpoints included the study of factors associated with changes in TC and fraction at 6 months, and changes in TC and fractions between the first and last available visits.
Results: 85 RA patients (68 women, 80%) met the inclusion criteria, with a mean age of 59± 13 years and a mean disease duration of 19.5± 12.4 years; 73 (86%) and 76 (89%) patients were rheumatoid factor and ACPA positive respectively, and 66 (78%) had bone erosions. 22 patients received baricitinib, 7 tofacitinib, 38 upadacitinib and 18 filgotinib. The DAS28-CRP at inclusion was 4.09± 1.26 with a mean CRP of 10±14 mg/L. Comorbidities, concomitant treatments and lipid profile at inclusion are presented in Tables 1 and 2. At inclusion, TC and HDL cholesterol (HDL-C) levels correlated negatively with CRP (respectively r=-0.23, p=0.042 and r=-0.25, p=0.040), but not with DAS28 or DAS28-CRP. This correlation was not observed for LDL and triglycerides.At 6 months, there was a significant increase in TC (+0.17±0.37 g/L, p=0.016) and HDL-C (+0.09±0.19g/L, p=0.017), but not LDL-C (+0.02±0.31, p=0.68) (Table 2). This increase was inversely correlated with the reduction in CRP concentrations (-6±10 mg/L) upon JAKi (TC: r=-0.31, p=0.033 and HDL-C: r=-0.32, p=0.042). This increase was not associated with the use of corticosteroids or methotrexate, nor with the course of the number of tender and swollen joints. At the last available visit (20±15 months after the onset of JAKi), there was a drop in TC and particularly in its HDL fraction compared with the first visit, with values close to those at the inclusion visit (Table 2). Statins were prescribed for 9 patients during the follow-up period, all of whom had known dyslipidemia at inclusion (10.6%).
Conclusion: This study confirms a transient increase in TC and TC fractions under JAKi, linked to the drug-induced reduction in systemic inflammation. These lipid abnormalities were regressive in the majority of patients. The clinical consequences of this elevation remain to be determined in the context of the cardiovascular tolerance of JAKi.
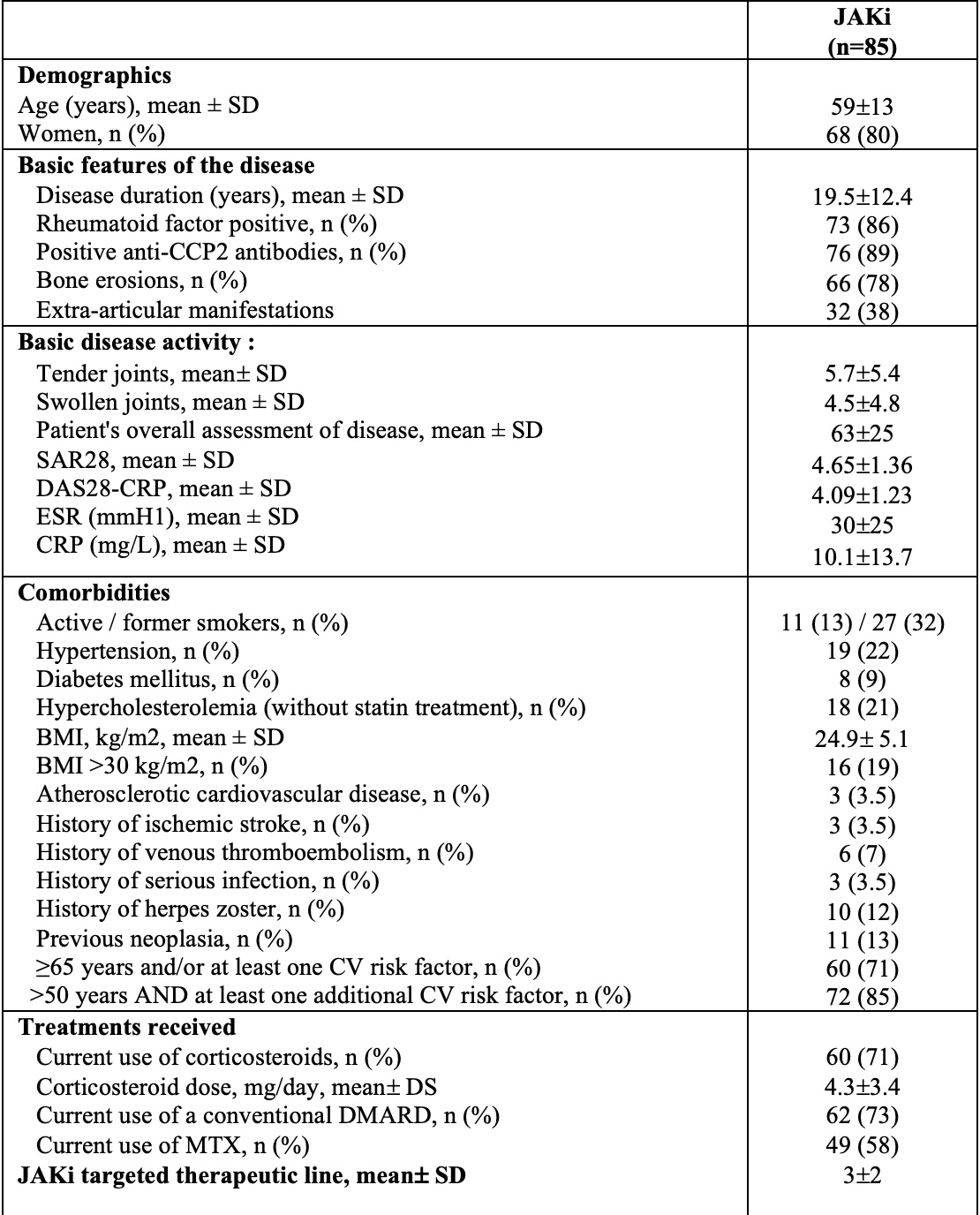 Table 1: Patient characteristics
Table 1: Patient characteristics
.jpg) Table 2: Changes in total cholesterol and fractions
Table 2: Changes in total cholesterol and fractions
To cite this abstract in AMA style:
Abdellaoui S, Al Tabaa O, Hecquet S, Combier A, Carves S, Fogel O, Allanore Y, Avouac J. Assessment of lipid abnormalities after initiation of a JAK inhibitor in rheumatoid arthritis in clinical practice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/assessment-of-lipid-abnormalities-after-initiation-of-a-jak-inhibitor-in-rheumatoid-arthritis-in-clinical-practice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-lipid-abnormalities-after-initiation-of-a-jak-inhibitor-in-rheumatoid-arthritis-in-clinical-practice/
