Session Information
Date: Monday, October 27, 2025
Title: (1191–1220) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Inclusion body myositis (IBM) is a progressive muscle disorder characterized by inflammation and degeneration with altered proteostasis. To better understand the interrelationship between these two features, we aimed at establishing a novel preclinical IBM model.
Methods: First, we used quantitative PCR to determine expression of pro-inflammatory chemo- and cytokines including lymphotoxin (LT)-signaling pathway components in human skeletal muscle tissue diagnosed with myositis. Based on these results we generated a mouse model that we analyzed at the histological, ultrastructural, transcriptional, biochemical, and behavioural level. Lastly, we subjected this model to anti-inflammatory treatments.
Results: After confirming and extending previous data on activation of lymphotoxin (LT)-signaling in human myositis, we generated distinct transgenic mouse lines co-expressing LTa and -b in skeletal muscle fibers. Transgenic mice displayed chronic myositis accompanied by dysregulated proteostasis, including an altered autophagolysosomal pathway. Related genes were temporarily up- and later downregulated, possibly in a compensatory fashion. Therefore, we genetically impaired autophagy in skeletal muscle cells. Autophagy impairment alone induced a pro-inflammatory transcriptional state, but no obvious cellular inflammation. However, the combination of LT-driven myositis with autophagy impairment induced the full spectrum of characteristic molecular and pathological features of IBM in skeletal muscle, including protein aggregates with typical ultrastructural morphology and mild mitochondrial pathology. Our attempts to treat the pathology by subjecting these mice to corticosteroids or anti-Thy1.2 antibodies mirrored recent treatment failures in humans, i.e., none of these treatments resulted in significant clinical improvement of motor performance or the transcriptional profile of muscle pathology.
Conclusion: In summary, these data provide evidence that inflammation and autophagy disruption play a synergistic role in the development of IBM-like muscular pathology. Furthermore, once established, IBM-like pathology in these mice, as in human IBM patients cannot be reverted or prevented from progression by conventional means of immunosuppression. We expect that this novel mouse model will help to identify future treatment modalities for IBM.
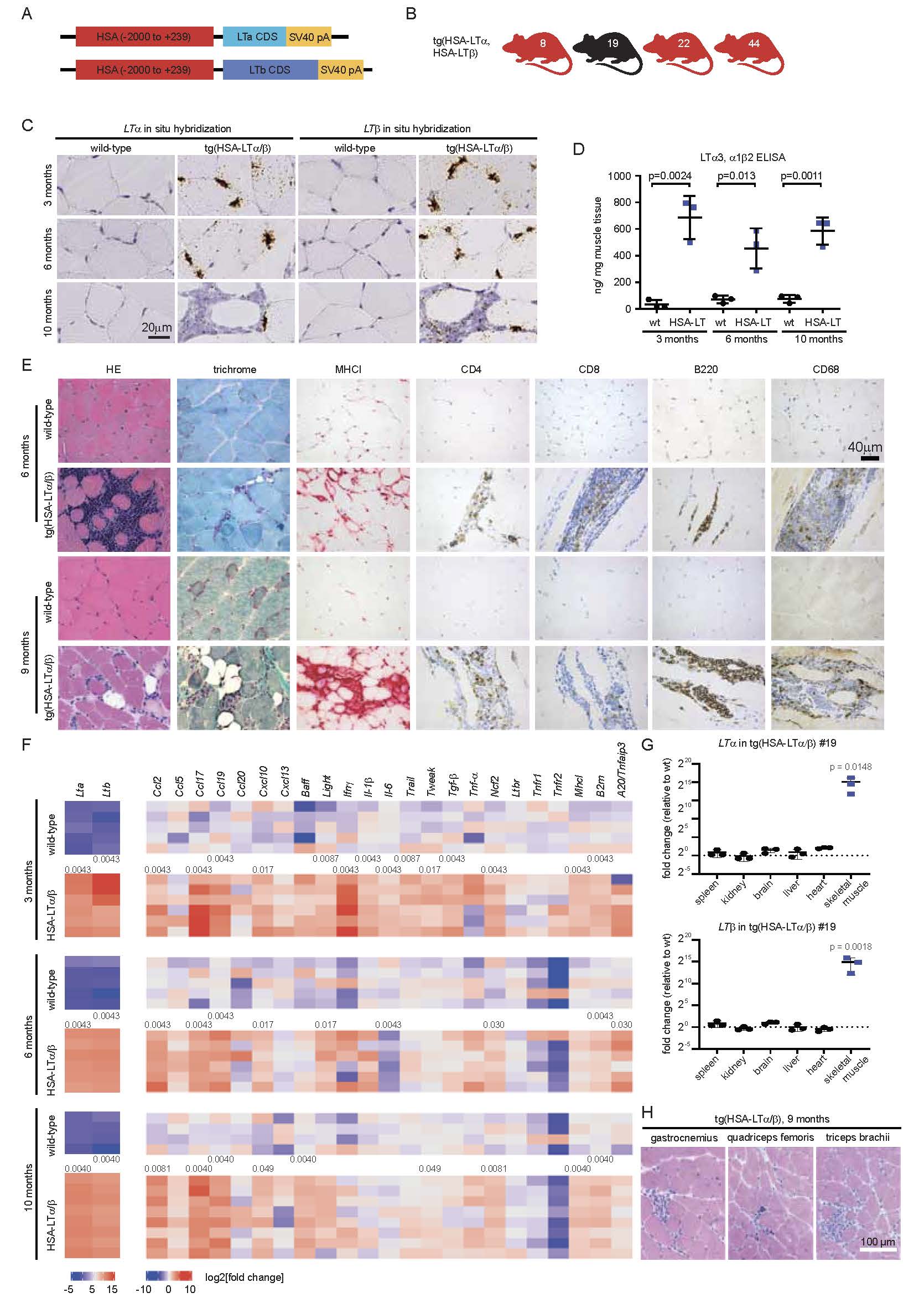 Figure 1. Generation, histology and chemo-/cytokine expression of HSA-LT/ transgenic mice. (A) Schematic drawing of the transgenic constructs of the coding sequences (CDS) of LT and LT both being cloned downstream of the human skeletal muscle actin (HSA) promoter (-2000 to +239) followed by an SV40 poly A site. (B) Four founder mice carrying both transgenes, HSA-LT and HSA-LT and transmitting them through the germline were obtained (line #8, #19, #22, #44). (C-D) LT and LTtransgene expression was detected in quadriceps muscle fibers of HSA-LT/ #19 mice by RNA in situ hybridization of paraffin sections (brown signal) in the muscle fibers at 3, 6 and 10 months of age and also in inflammatory infiltrates in skeletal muscle, shown at 10 months of age (C). LT ELISA with skeletal muscle tissue homogenate from indicated time points and genotypes. Data are shown as ng LT3/LT12/LT21 protein per mg total protein. Statistical significance was tested using two-tailed Student’s t-test. (E) Histologically, HSA-LT/ transgenic mice show endomysial inflammatory infiltrates and occasional muscle fiber necrosis. Especially at the age of 9 months, there is fibrosis and partial replacement by fatty tissue within the endomysium. Fiber size variation is also increased in transgenic mice (H&E and trichrome). In addition to the normal MHCI localization on endomysial capillaries seen in wild-type mice, transgenic mice display focal sarcolemmal MHCI upregulation (red signal). The inflammatory infiltrates are mostly composed of B220+ B cells and CD68+ macrophages and – to a lesser extent – of CD4+ and CD8+ T cells (brown signals). (F) Heatmap of chemo- and cytokine expression in HSA-LT/ transgenic mice determined by quantitative PCR of quadriceps femoris muscle tissue at 3, 6 and 10 months of age. P-values were determined using two-tailed Mann-Whitney tests. In case of significant differences between wild-type and transgenic mice, the p-values are displayed between the group of wild-type and transgenic line of the respective age group. (G) Quantitative PCR determining LTand LT expression in different organs of HSA-LT/ transgenic mice. P-values were determined using the ANOVA test with Šídák’s multiple comparison test, significant differences compared to wild-type are displayed. (H) HE-stained sections of three different muscles from 9 months old HSA-LT/mice.
Figure 1. Generation, histology and chemo-/cytokine expression of HSA-LT/ transgenic mice. (A) Schematic drawing of the transgenic constructs of the coding sequences (CDS) of LT and LT both being cloned downstream of the human skeletal muscle actin (HSA) promoter (-2000 to +239) followed by an SV40 poly A site. (B) Four founder mice carrying both transgenes, HSA-LT and HSA-LT and transmitting them through the germline were obtained (line #8, #19, #22, #44). (C-D) LT and LTtransgene expression was detected in quadriceps muscle fibers of HSA-LT/ #19 mice by RNA in situ hybridization of paraffin sections (brown signal) in the muscle fibers at 3, 6 and 10 months of age and also in inflammatory infiltrates in skeletal muscle, shown at 10 months of age (C). LT ELISA with skeletal muscle tissue homogenate from indicated time points and genotypes. Data are shown as ng LT3/LT12/LT21 protein per mg total protein. Statistical significance was tested using two-tailed Student’s t-test. (E) Histologically, HSA-LT/ transgenic mice show endomysial inflammatory infiltrates and occasional muscle fiber necrosis. Especially at the age of 9 months, there is fibrosis and partial replacement by fatty tissue within the endomysium. Fiber size variation is also increased in transgenic mice (H&E and trichrome). In addition to the normal MHCI localization on endomysial capillaries seen in wild-type mice, transgenic mice display focal sarcolemmal MHCI upregulation (red signal). The inflammatory infiltrates are mostly composed of B220+ B cells and CD68+ macrophages and – to a lesser extent – of CD4+ and CD8+ T cells (brown signals). (F) Heatmap of chemo- and cytokine expression in HSA-LT/ transgenic mice determined by quantitative PCR of quadriceps femoris muscle tissue at 3, 6 and 10 months of age. P-values were determined using two-tailed Mann-Whitney tests. In case of significant differences between wild-type and transgenic mice, the p-values are displayed between the group of wild-type and transgenic line of the respective age group. (G) Quantitative PCR determining LTand LT expression in different organs of HSA-LT/ transgenic mice. P-values were determined using the ANOVA test with Šídák’s multiple comparison test, significant differences compared to wild-type are displayed. (H) HE-stained sections of three different muscles from 9 months old HSA-LT/mice.
.jpg) Figure 2. Behavioural and histological consequences of autophagy depletion in lymphotoxin-induced chronic myositis. (A) Breeding scheme to obtain HSA-LT/+ Ckmm-Cre+ Atg5fl/fl (HSA-LT;CreAtg5) mice. (B-C) Muscle volume was determined by magnetic resonance imaging (MRI) at 3 and 6 months of age of male and female HSA-LT;CreAtg5 compared to Ckmm-Cre+ Atg5fl/fl (CreAtg5) mice. Representative MRI (FLASH) images and 3D reconstructions of calf muscles are shown along with volumetric quantification. Muscle atrophy was observed in HSA-LT;CreAtg5 compared to CreAtg5 mice. Two-sided unpaired student‘s t-test was used to determine p-values. (D-F) Histological and immunohistochemical analysis for inflammatory markers revealed chronic myositis in HSA-LT;CreAtg5, but not in CreAtg5 mice, characterized by endomysial infiltrates composed of CD4+ and CD8+ T cells, B220+ B cells and CD68+ macrophages (brown signals) as previously observed in HSA-LT/ transgenic mice. Sarcolemmal upregulation of MHCI were detected in HSA-LT;CreAtg5, but MHCI was either not (1 of 4) or expressed weakly/ only on single fibers (3 of 4) in CreAtg5 mice (red signal). Quantification is shown in F. P-values were determined using the ANOVA test with Šídák’s multiple comparison test. (G-H) Motor performance of CreAtg5 and HSA-LT;CreAtg5 mice was determined by the grip strength test (G) and by the hanging wire test (H). Groups were compared using the two-sided unpaired student‘s t-test. P-values are shown.
Figure 2. Behavioural and histological consequences of autophagy depletion in lymphotoxin-induced chronic myositis. (A) Breeding scheme to obtain HSA-LT/+ Ckmm-Cre+ Atg5fl/fl (HSA-LT;CreAtg5) mice. (B-C) Muscle volume was determined by magnetic resonance imaging (MRI) at 3 and 6 months of age of male and female HSA-LT;CreAtg5 compared to Ckmm-Cre+ Atg5fl/fl (CreAtg5) mice. Representative MRI (FLASH) images and 3D reconstructions of calf muscles are shown along with volumetric quantification. Muscle atrophy was observed in HSA-LT;CreAtg5 compared to CreAtg5 mice. Two-sided unpaired student‘s t-test was used to determine p-values. (D-F) Histological and immunohistochemical analysis for inflammatory markers revealed chronic myositis in HSA-LT;CreAtg5, but not in CreAtg5 mice, characterized by endomysial infiltrates composed of CD4+ and CD8+ T cells, B220+ B cells and CD68+ macrophages (brown signals) as previously observed in HSA-LT/ transgenic mice. Sarcolemmal upregulation of MHCI were detected in HSA-LT;CreAtg5, but MHCI was either not (1 of 4) or expressed weakly/ only on single fibers (3 of 4) in CreAtg5 mice (red signal). Quantification is shown in F. P-values were determined using the ANOVA test with Šídák’s multiple comparison test. (G-H) Motor performance of CreAtg5 and HSA-LT;CreAtg5 mice was determined by the grip strength test (G) and by the hanging wire test (H). Groups were compared using the two-sided unpaired student‘s t-test. P-values are shown.
.jpg) Figure 3. IBM-like myositis in mice is resistant to anti-inflammatory treatments. (A) Timeline of treatment. Density of CD3+ T cells in the liver of untreated mice and after treatment; for anti-Thy1.2 (B) and prednisolone (C) treatment. P-values were determined using two-tailed Students t-tests. Grip strength of mice at day 150. The results show genotype-dependent differences, but no treatment effect after prednisolone (D) and anti-Thy1.2 (E) treatment. (F) The heatmap shows expression examined by qPCR relative to the respective untreated controls (treated compared to untreated wild-type, HSA-LT/and HSA-LT;CreAtg5, respectively) – here in case of prednisolone treatment. Except for a significant downregulation of Cxcl10, Ncf2 and Gpx2 following prednisolone treatment of HSA-LT/mice compared to untreated HSA-LT/and non-significant trends for some other genes, there was a counterintuitive upregulation of Ccl5 in treated compared to untreated HSA-LT;CreAtg5 mice, but otherwise stable gene expression. n.d. = not determined. Mann-Whitney-U test was performed as statistical test since some values were not normally distributed. P-values are only displayed in case of statistical significance between untreated and treated mice. Behavioral phenotypes and gene expression patterns following these treatments were stable (see also Supplementary figures).
Figure 3. IBM-like myositis in mice is resistant to anti-inflammatory treatments. (A) Timeline of treatment. Density of CD3+ T cells in the liver of untreated mice and after treatment; for anti-Thy1.2 (B) and prednisolone (C) treatment. P-values were determined using two-tailed Students t-tests. Grip strength of mice at day 150. The results show genotype-dependent differences, but no treatment effect after prednisolone (D) and anti-Thy1.2 (E) treatment. (F) The heatmap shows expression examined by qPCR relative to the respective untreated controls (treated compared to untreated wild-type, HSA-LT/and HSA-LT;CreAtg5, respectively) – here in case of prednisolone treatment. Except for a significant downregulation of Cxcl10, Ncf2 and Gpx2 following prednisolone treatment of HSA-LT/mice compared to untreated HSA-LT/and non-significant trends for some other genes, there was a counterintuitive upregulation of Ccl5 in treated compared to untreated HSA-LT;CreAtg5 mice, but otherwise stable gene expression. n.d. = not determined. Mann-Whitney-U test was performed as statistical test since some values were not normally distributed. P-values are only displayed in case of statistical significance between untreated and treated mice. Behavioral phenotypes and gene expression patterns following these treatments were stable (see also Supplementary figures).
To cite this abstract in AMA style:
Bremer J, Nagel J, Zschüntzsch J, Zajt K, Palaz T, Blank T, Ikis A, Fischer L, Sensmeyer A, Wiechers L, Reichelt J, Hofmann K, Wolf M, Leuchtenberger C, Tripathi P, Einer C, Zischka H, Rothermel U, Eck A, Reimann R, Kana V, Rushing E, Aguzzi A, Prinz M, Liebetanz D, Odoardi F, Kuo C, Weis J, Kraft F, Schmidt J, Heikenwälder M. Overexpression of lymphotoxin in autophagy-deficient mice as model for inclusion body myositis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/overexpression-of-lymphotoxin-in-autophagy-deficient-mice-as-model-for-inclusion-body-myositis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/overexpression-of-lymphotoxin-in-autophagy-deficient-mice-as-model-for-inclusion-body-myositis/
