Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Dose reduction of biologic therapy (BT) in patients with rheumatoid arthritis (RA) and spondyloarthritis (SpA) has been increasingly implemented in recent years, in accordance with international and local clinical practice guidelines. This approach seeks to minimize the risk of adverse events and reduce direct healthcare costs. The objective of this study is to describe clinical characteristics and identify factors associated with dose reduction and discontinuation of BT in a cohort of RA and SpA patients managed within a comprehensive rheumatology care program at the healthcare institution Riesgo de Fractura SA – CAYRE IPS. Additionally, to evaluate direct pharmacologic treatment costs in this population.
Methods: This real-world evidence study describes a cohort of adult RA and SpA patients in sustained clinical remission—defined as DAS28 < 2.6 for RA and ASDAS < 1.3 for SpA—for at least 12 months. Patients were followed from April 2023 to October 2024. Clinical outcomes (DAS28 or ASDAS) were assessed at 6, 12, and 18 months after initiating biologic dose reduction. Per institutional policy, Rituximab and Adalimumab were used as first-line BT based on cost and biosimilar availability. Logistic regression was used to identify factors associated with successful dose reduction. Variables were selected based on clinical relevance and prior evidence; the most parsimonious model was applied. Limitations included small sample size and a lack of heterogeneity between groups. Drug costs were estimated using SISPRO platform by averaging unit prices, and all analyses were conducted using R software. Costs are reported in USD using an exchange rate of 1 USD = COP 4,293.00 (official Colombian rate (average), October 2024).
Results: This cohort includes 1,456 patients with RA, of whom 402 are receiving BT, and 229 patients with SpA, of whom 146 are receiving BT. Over 18 months, BT doses were reduced in 65.9% (265) of RA patients and 50.6% (74) of SpA patients. BT was discontinued in 26 RA and 3 SpA patients. In RA, biologic dose reduction occurred in 58% of first-line and 24% of second-line therapies, with a failure rate of 17%. In SpA, reductions occurred in 65% of first-line and 24% of second-line therapies, with a 21% failure rate. Cost savings from dose reduction over 18 months were $854,874.8 for RA and $220,551.5 for SpA. Logistic regression was performed to identify variables associated with successful dose reduction. No statistically significant factors were found in RA. In SpA, shorter disease duration (< 5 years) was associated with lower odds of optimization (OR 0.40; 95% CI: 0.19–0.86; p=0.0192). HLA-B27 positivity was not significant but showed a trend toward lower odds of optimization (OR < 1, p = 0.1118).
Conclusion: This real-world study within a comprehensive care model showed that tapering or discontinuing biologic therapy in RA and SpA patients is feasible while maintaining disease control and achieving substantial cost savings, with a favorable economic impact on the healthcare system. Larger studies are needed to better identify factors associated with successful dose reduction or discontinuation of biologics.
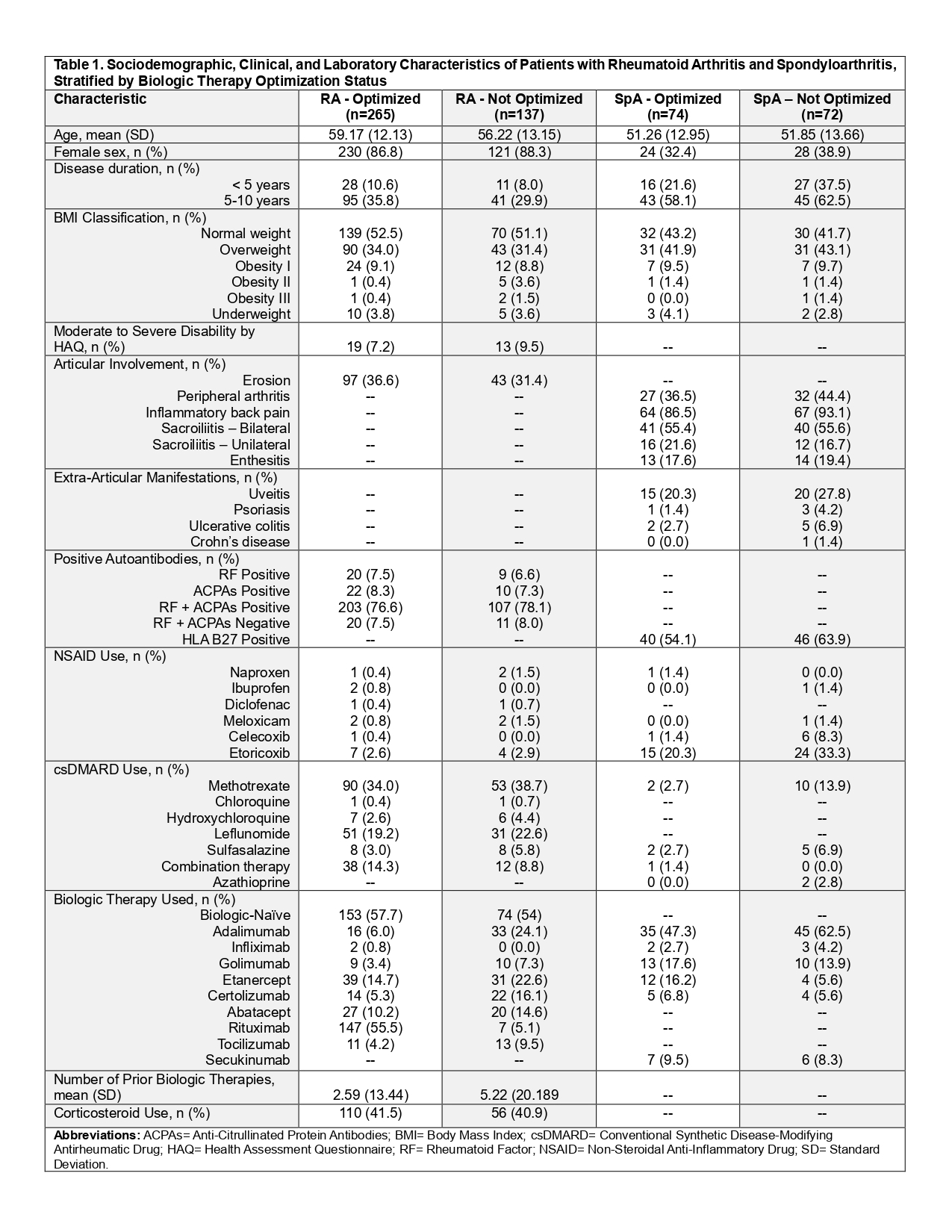 Table 1. Sociodemographic, Clinical, and Laboratory Characteristics of Patients with Rheumatoid Arthritis and Spondyloarthritis, Stratified by Biologic Therapy Optimization Status
Table 1. Sociodemographic, Clinical, and Laboratory Characteristics of Patients with Rheumatoid Arthritis and Spondyloarthritis, Stratified by Biologic Therapy Optimization Status
.jpg) Table 2. Multivariable Logistic Regression Model for Biologic Therapy Optimization in Spondyloarthritis
Table 2. Multivariable Logistic Regression Model for Biologic Therapy Optimization in Spondyloarthritis
.jpg) Figure 1. Disease Activity by DAS28 and ASDAS at 6, 12, and 18 Months According to Disease Type.
Figure 1. Disease Activity by DAS28 and ASDAS at 6, 12, and 18 Months According to Disease Type.
To cite this abstract in AMA style:
Jauregui E, Restrepo D, Garzon B, Atuesta A, Correa N, Barrera J, Rubio A. Associated Factors And Direct Cost Analysis Of Dose Reduction And Discontinuation Of Biological Therapies In Rheumatoid Arthritis And Spondyloarthritis: Findings From A Colombian Cohort Within The Framework Of An Integrated Management Model [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/associated-factors-and-direct-cost-analysis-of-dose-reduction-and-discontinuation-of-biological-therapies-in-rheumatoid-arthritis-and-spondyloarthritis-findings-from-a-colombian-cohort-within-the-fra/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associated-factors-and-direct-cost-analysis-of-dose-reduction-and-discontinuation-of-biological-therapies-in-rheumatoid-arthritis-and-spondyloarthritis-findings-from-a-colombian-cohort-within-the-fra/
