Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To develop effective novel treatment strategies for Gout disease that cater to patients from diverse backgrounds, it is crucial that all racial groups, without exception, have equal opportunities to participate in clinical trials. This inclusivity is not just a matter of fairness, but a necessity for the advancement of medical research.
Methods: Using ClinicalTrials.gov, a web-based resource that registers all studies meeting the definition of a clinical trial according to the International Committee on Medical Journal Editors,we identified all phase 1 to phase 4 Gout disease clinical trials. We manually abstracted data on the racial distribution of enrolled participants, sex distribution, trial phase, location, and year of trial reporting. Additionally, we conducted subgroup analyses of racial distribution based on trial phase and location and evaluated trends in racial disparity within clinical trial enrollment over the years. SPSS software version 28 was used for analysis. P value of < 0.05 was considered statistically significant.
Results: We identified 78 clinical trials related to Gout, of which 91.7% were done in Europe or the United States. Most of these were phase 2 or 3 clinical trials (70.4%). The racial distribution of enrolled participants was publicly available in 44 (56.4%) clinical trials. The race distribution of enrolled subjects was reported in 33.3% of Phase 1, 60.9% in Phase 2, 48.1% in Phase 3 and 53.3% in Phase 4. The race distribution among 29,166 study participants in 78 clinical trials was: 61.3% White race (14522), 15.8 % Black race (3738), 6.1% Asians descent (1450), 9.2% Hispanics origin (2189), 2.4% American Indian/Alaskan Native (559), 0.8% Native Hawaiian/Other Pacific Islander (198), and 4.3% other races (1029). A similar race distribution trend was observed across all subgroups of clinical trials based on phase. There was a statically significant difference between trail phases regarding racial groups with p < 0.001. The sex distribution, as seen throughout the trials, was 87.7% in males (25572) and 12.3% in females (3594). There was a statistically significant difference between trial phases regard toregarding sex at birth with p < 0.001.
Conclusion: Our research revealed that only 56.4% of Gout studies reported racial distribution. The Black race was underrepresented despite a higher prevalence of Gout in this population per the literature evidence. Strategies to ensure equitable representation in Gout clinical trials are needed to address disparities and improve treatment outcomes across diverse populations.
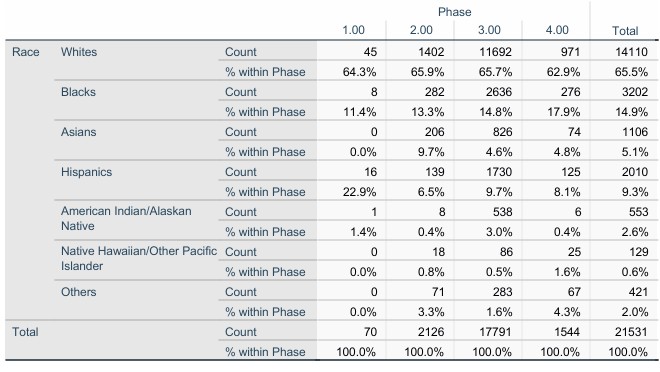 Race Distribution of Participants Across Clinical Trial Stages (Stage 1 to 4)
Race Distribution of Participants Across Clinical Trial Stages (Stage 1 to 4)
.jpg) Gender Distribution of Participants Across Clinical Trial Stages (Stage 1 to 4)
Gender Distribution of Participants Across Clinical Trial Stages (Stage 1 to 4)
To cite this abstract in AMA style:
Zulfiqar F, Kaur D, Bethea M, Spencer T, Bitla S, Vyas A, Arsene C. Racial and Gender Disparities in Gout Clinical Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/racial-and-gender-disparities-in-gout-clinical-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/racial-and-gender-disparities-in-gout-clinical-trials/
