Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Observational studies investigating glucocorticoids (GCs) and related adverse events (AEs) in patients with rheumatoid arthritis (RA) can suffer from bias by indication if confounders such as disease activity or systemic inflammation are not adjusted for in the statistical analyses. We assessed whether observational studies in RA which investigate the relationship between GC use and AEs regularly adjust for disease activity or systemic inflammation.
Methods: We conducted a protocolized (https://dx.doi.org/10.17504/protocols.io.bp2l6x955lqe/v1) systematic literature review. MEDLINE was searched for observational studies published since 2008 in patients with RA that investigated the association between GCs and certain AEs. GCs had to be mentioned in the study abstract. We calculated the proportion of studies that statistically adjusted for validated disease activity scores (e.g., RAPID3) and/or systemic inflammation (e.g., serum CRP levels) and/or both (e.g., both RAPID3 and CRP or combined scores such as DAS28-CRP). Joanna Briggs Institute’s critical appraisal checklists were used for quality appraisal. Analyses were further stratified by study characteristics (year of publication, study type, type of AE, critical appraisal checklist score percentages, and 2022 Journal Impact Factor®). Statistical comparisons were made with Chi-squared tests.
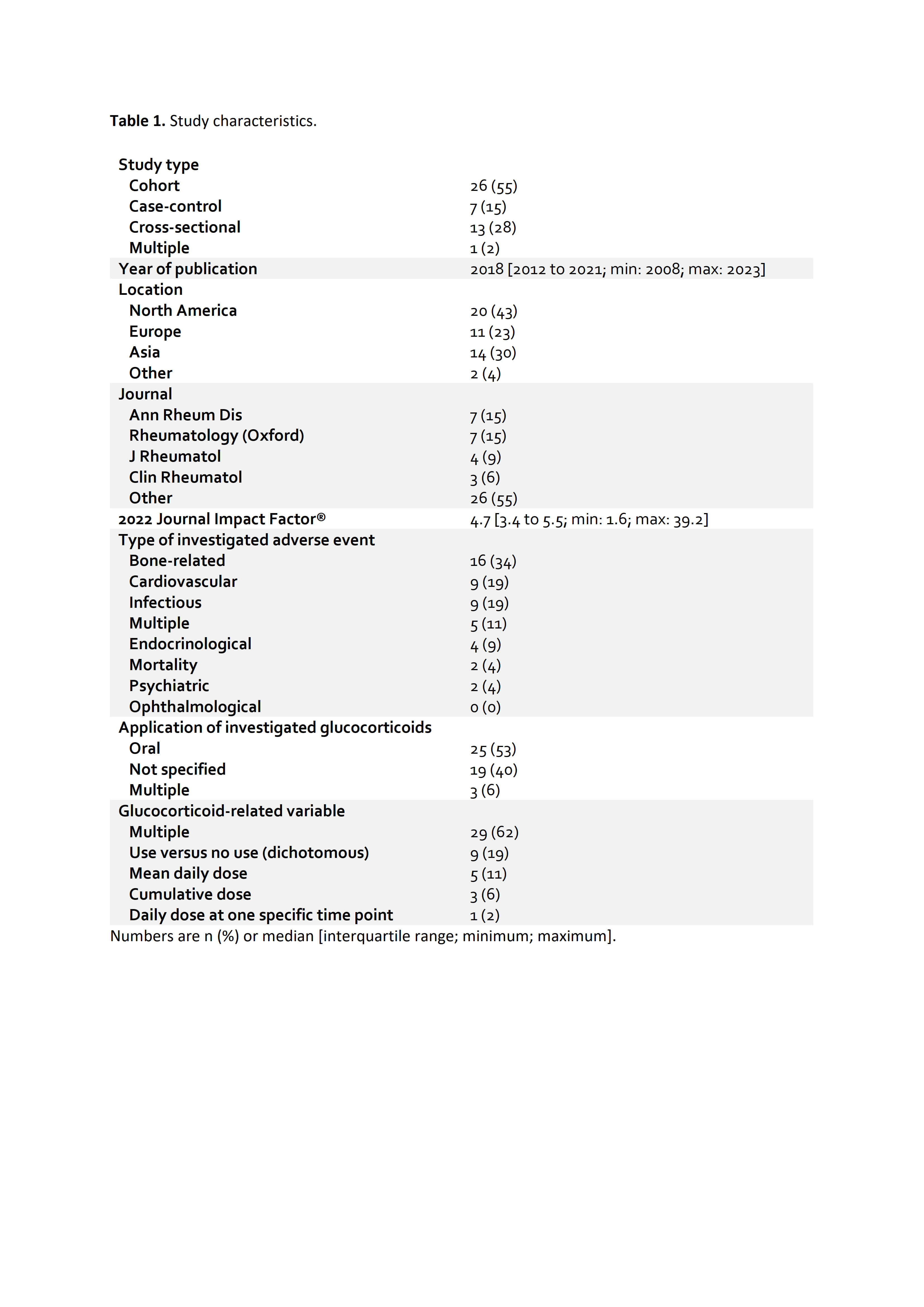
Results: Out of 609 initial search results, 47 observational (mostly cohort) studies were finally included (Table 1). Most studies had a cohort design, were from North America or Europe, and assessed oral GCs. The most commonly investigated AEs were bone-related such as bone mineral density or fractures, followed by cardiovascular and infectious AEs. Almost one third of all included studies were published in either Annals of the Rheumatic Diseases or Rheumatology (Oxford). Most studies (57%) neither adjusted for validated disease activity scores nor markers of systemic inflammation, regardless of study characteristics (Figure 1). Even in the subsets of studies that were published in a journal with a Journal Impact Factor® of ≥10 or that had critical appraisal checklist score percentages of ≥90%, the majority of studies did not statistically adjust for disease activity or markers of inflammation.
Conclusion: Confounding by indication is likely present in most observational studies investigating the relationship between GC use and AEs. Even studies published in “high-ranking” scientific journals did not regularly adjust for disease activity or markers of systemic inflammation. Observational studies may attain relatively high quality appraisal scores according to a highly regarded, commonly used critical appraisal tool despite not accounting for the possibility of confounding by indication.
To cite this abstract in AMA style:
Palmowski A, Beenken A, Pankow A, Oldenkott J, Käding H, Wiebe E, Boyadzhieva Z, Matteson E, Minopoulou I, Witte T, Simon D, Kleyer A, BUTTGEREIT F. Confounding by Indication in Observational Studies Investigating Glucocorticoid-Associated Adverse Events in Patients with Rheumatoid Arthritis: A Systematic Literature Review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/confounding-by-indication-in-observational-studies-investigating-glucocorticoid-associated-adverse-events-in-patients-with-rheumatoid-arthritis-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/confounding-by-indication-in-observational-studies-investigating-glucocorticoid-associated-adverse-events-in-patients-with-rheumatoid-arthritis-a-systematic-literature-review/

.jpg)