Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Hypertension was a common adverse event in RCTs testing voclosporin, an oral calcineurin inhibitor approved in 2021 to treat lupus nephritis (LN). In these trials, increases in blood pressure (BP) were generally transitory and frequently diminished after the first 4 weeks of use. Here we characterize BP changes in real-world data.
Methods: Data came from RISE, a national registry with electronic health records from over 1100 US rheumatologists. Voclosporin use was abstracted from medication tables. All systolic and diastolic BPs from 60 days before the first order to 90 days after the last order were tabulated. Baseline BP was defined as the lowest BP before the index date (inclusive) and categorized as follows: normal < 130 mmHg AND < 80 mmHg; elevated, 130-164 mmHg OR 80-104 mmHg; and high, ≥165 mmHg OR ≥105 mmHg. Covariates included age, sex, race and ethnicity, insurance coverage, area deprivation index, BMI, smoking status, and time-varying antihypertensive drug use.We describe patient characteristics overall and stratified by whether the patient experienced an 8 mmHg increase in systolic BP (minimum clinically important difference (MCID)) during the first year of use. We calculated the median time to a MCID within a year for both systolic and diastolic using Kaplan Meier estimates. Finally, we present mean changes in BP between baseline and the lowest BP reading in windows of 0-1, 1-2, 2-3, 3-6, 6-9, and 9-12 months after the index date.
Results: We included 287 patients: 83.6% were female, 32% were black; mean (SD) age was 41.4 (±14.1) years (Table 1). The mean systolic BP at baseline was 125.7 (±16.1)/79.2 (±10.8) (42.2% categorized as normal, 54% elevated, and 3.8% high). At baseline, 67.3% were on an antihypertensive medication. Overall, 63.8% experienced a systolic MCID, and 49.1% a diastolic MCID. The median time to a recorded increase in systolic was 77 days (95%CI: 62-91) (Figure 1), and 134 days (95%CI: 99-198) for diastolic. The average systolic/diastolic BP change was largest in the first month for patients with normal baseline BP 13.7 (±18.6)/6.0 (±13.8), and less pronounced for those with elevated BP 2.7 (±18.8)/-0.6 (±11.2); BP decreased for those with high baseline BP -7.9 (±17.8)/-7.3 (±16.5) (Figure 2). BPs were stable over time for those with baseline normal BP whereas BPs returned to baseline levels after 6 months for patients with elevated baseline BPs. Although the sample size was small for patients with high baseline BPs we observed large decreases in BP during the first 3 months of use, likely due to the beneficial effects of voclosporin on underlying LN.
Conclusion: >50% of patients using voclosporin had a MCID in the first year of use. Increases tend to occur in the first 3 months of use and on average were larger for patients with normal baseline BPs. In contrast to findings reported from RCTs, BP increases for patients with normal baseline BPs were persistent over 12 months. Although the MCID may seem small, it has been associated with up to 20% increase in the risk of cardiovascular disease (CVD) events. The clinical significance of BP changes related to voclosporin use warrant further investigation, especially since people with LN are already at increased risk for CVD.
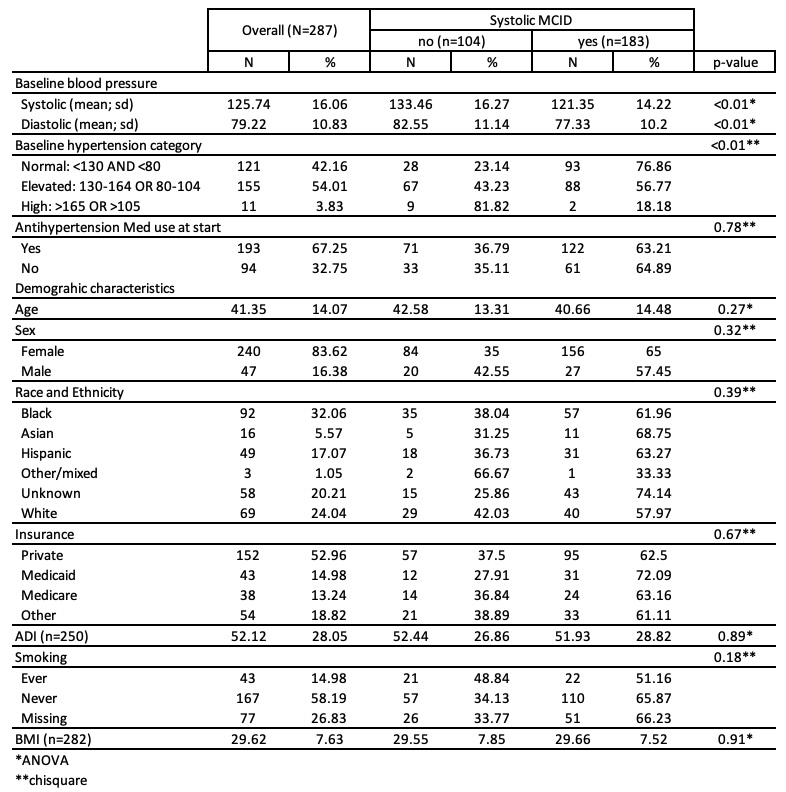 Table 1. Demographic and clinical characteristics of voclosporin patients in RISE overall and stratified by experiencing an 8 mmHg increase in systolic blood pressure during the first year of use.
Table 1. Demographic and clinical characteristics of voclosporin patients in RISE overall and stratified by experiencing an 8 mmHg increase in systolic blood pressure during the first year of use.
.jpg) Figure 1. Kaplan Meier curve showing the time to an MCID in systolic blood pressure of 8 mmHg after initiating voclosporin.
Figure 1. Kaplan Meier curve showing the time to an MCID in systolic blood pressure of 8 mmHg after initiating voclosporin.
.jpg) Figure 2. Box and whisker plots of mean change in systolic and diastolic blood pressure between baseline and the specified time stratified by patient’s baseline hypertension category.
Figure 2. Box and whisker plots of mean change in systolic and diastolic blood pressure between baseline and the specified time stratified by patient’s baseline hypertension category.
To cite this abstract in AMA style:
Roberts E, Schmajuk g, Yazdany J. Blood pressure remains elevated in patients initiating voclosporin with low baseline blood pressure: evidence from patients in routine clinical care [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/blood-pressure-remains-elevated-in-patients-initiating-voclosporin-with-low-baseline-blood-pressure-evidence-from-patients-in-routine-clinical-care/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/blood-pressure-remains-elevated-in-patients-initiating-voclosporin-with-low-baseline-blood-pressure-evidence-from-patients-in-routine-clinical-care/
