Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The newly published ACR guidelines recommend initiating triple therapy for all patients with proliferative lupus nephritis (LN). While belimumab (BEL) has demonstrated efficacy in clinical trials, real-world data on its early use in combination regimens remain limited.We aimed to evaluate the effectiveness of triple therapy including BEL plus standard of care (SoC) initiated within 6 months of LN onset in achieving renal response and glucocorticoid tapering in a real-world multicenter cohort.
Methods: Retrospective multicenter study in Spain including patients with proliferative LN (ISN/RPS class III, IV, or mixed III/IV+V) treated with BEL-based triple therapy within 6 months of diagnosis.
Results: A total of 38 patients were included (78.9% female, mean age 38±14 years); 68.4% were Caucasian and 21.1% Hispanic. A history of previous LN episodes was present in 38.5% of patients.Histological classification included class III (n=11), class IV (n=20), and mixed III/IV+V (n=7); two had thrombotic microangiopathy. Mean activity and chronicity indices were 9.4±4.0 and 2.3±1.9, respectively. All patients had a minimum follow-up of 6 months, with a median follow-up of 25 months (IQR 14.5–41). Induction therapy included intravenous methylprednisolone in 63.2%, with a mean initial oral prednisone dose of 35.7±17.9 mg/day. Most patients (76.3%) received MMF, while 23.7% were treated with cyclophosphamide. Maintenance therapy consisted of MMF in 94.7% of cases, with only two patients (5.3%) receiving azathioprine.CRR was achieved in 60% within 12 months; 65.7% achieved a primary response and 72.2% a partial response (Figure 1). Proteinuria decreased from 3.0±2.9 g/day at baseline to 560±466 mg/day at 12 months; eGFR remained stable, with progressive improvement from 71.3±30.3 to 78.4±23.1 mL/min/1.73m² at 12 months and 80.9 at 24 months (Table 1). Regarding glucocorticoid tapering, the 2023 ACR recommendations propose a target of ≤5 mg/day of prednisone by month 6. In our cohort, 65.8% of patients achieved this goal, with a mean dose of 6.0±3.2 mg/day at 6 months. Mean prednisone dose declined from 35.7±17.9 mg/day at baseline to 4.0±3.7 mg/day at 12 months. By month 12, 83.3% of patients were receiving ≤5 mg/day (Figure 2). At 24 months, mean prednisone was 3.6±5.0 mg/day (Table 1).Renal relapse occurred in 6 patients while on treatment with BEL (15.7%); BEL was discontinued in 2, while 4 continued BEL with intensified immunosuppression. Three patients experienced mild to moderate non-renal flares, managed with therapy adjustments (anifrolumab, methotrexate, or prednisone increase).When comparing early initiation of BEL ( < 3 months) versus delayed initiation (3–6 months post-flare), early use was associated with a significantly shorter time to achieve CRR (HR 4.7, 95% CI 1.6–13.5; p=0.04) and partial renal response (HR 2.47, 95% CI 1.1–5.8; p=0.037).
Conclusion: Initiation of triple therapy with BEL in proliferative LN was associated with robust renal responses and achievement of ACR steroid-sparing targets in most patients. Earlier treatment onset may further enhance renal outcomes
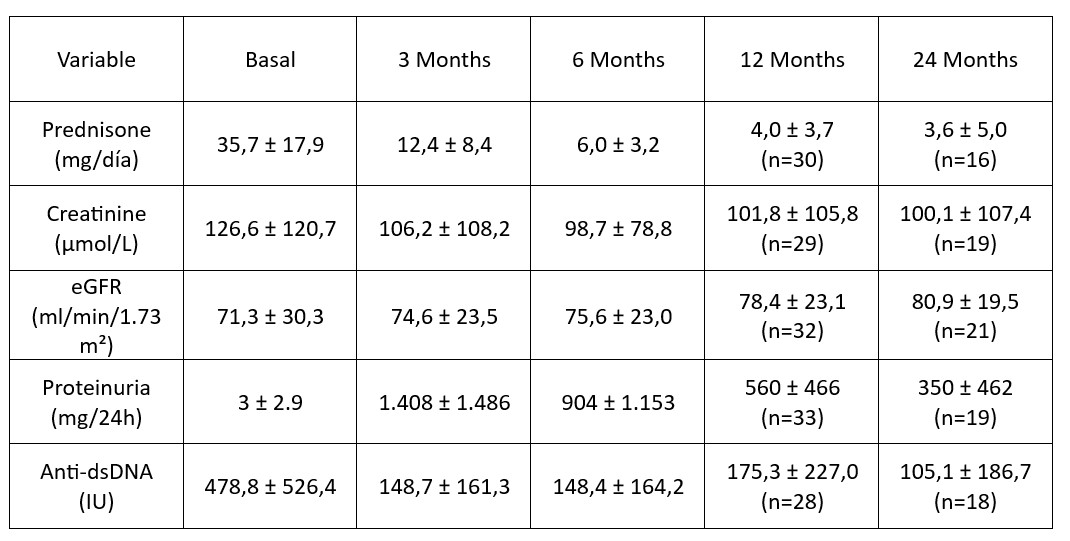 Table 1. Evolution of renal parameters over time
Table 1. Evolution of renal parameters over time
.jpg) Figure 1. Evolution of Renal Response Rates
Figure 1. Evolution of Renal Response Rates
.jpg) Figure 2. Proportion of Patients Achieving Glucocorticoid Tapering Targets
Figure 2. Proportion of Patients Achieving Glucocorticoid Tapering Targets
To cite this abstract in AMA style:
Vidal Montal P, Narváez J, Fabregat A, Rúa-Figueroa I, Pego-Reigosa J, Hernández-martín A, Moriano C, Garcia-Villanueva M, Garrote Corral S, Heredia S, Martínez Barrio J, Bernardez Moreno J, Vela Casasempere P, Tejera Segura B, Riancho L, Novoa F, Torrente-Segarra V, Piqueras García M, Frade Sosa B, Gomez-Puerta J, Ramos Giraldez C, Salman Montes T, Galindo-Izquierdo M, TOMERO MURIEL E, calvo J, Fragío Gil J, De la Rubia Navarro M, Magallares B, Altabás-González I. Belimumab-Based Triple Therapy in Proliferative Lupus Nephritis: Renal Outcomes and Glucocorticoid Tapering in a Real-World Multicenter Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/belimumab-based-triple-therapy-in-proliferative-lupus-nephritis-renal-outcomes-and-glucocorticoid-tapering-in-a-real-world-multicenter-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/belimumab-based-triple-therapy-in-proliferative-lupus-nephritis-renal-outcomes-and-glucocorticoid-tapering-in-a-real-world-multicenter-cohort/
