Session Information
Date: Sunday, October 26, 2025
Title: (0593–0640) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Herpes Zoster (shingles) is a common preventable adverse event in SLE. In Quebec, Canada, the Recombinant Zoster Vaccine (RZV) became free of charge for immunocompromised adults in May 2023. We evaluated RZV vaccination rates before and after this date, and examined factors associated with vaccination in SLE.
Methods: We studied participants from 2 prospective SLE cohorts who met ACR/SLICC classification criteria. Annual standardized data collection included demographics, clinical characteristics, medications, and vaccinations. Follow-up started at the first study visit after October 2017 (when RZV first became available), left-censoring person-time prior to the first study visit and excluding participants vaccinated before this date. Follow-up continued until RZV vaccination, death, end of cohort follow-up, or December 31, 2024. We compared vaccination rates before and after May 2023 (start of free vaccination), and evaluated demographic and clinical associations with vaccination using Cox proportional hazards regression.
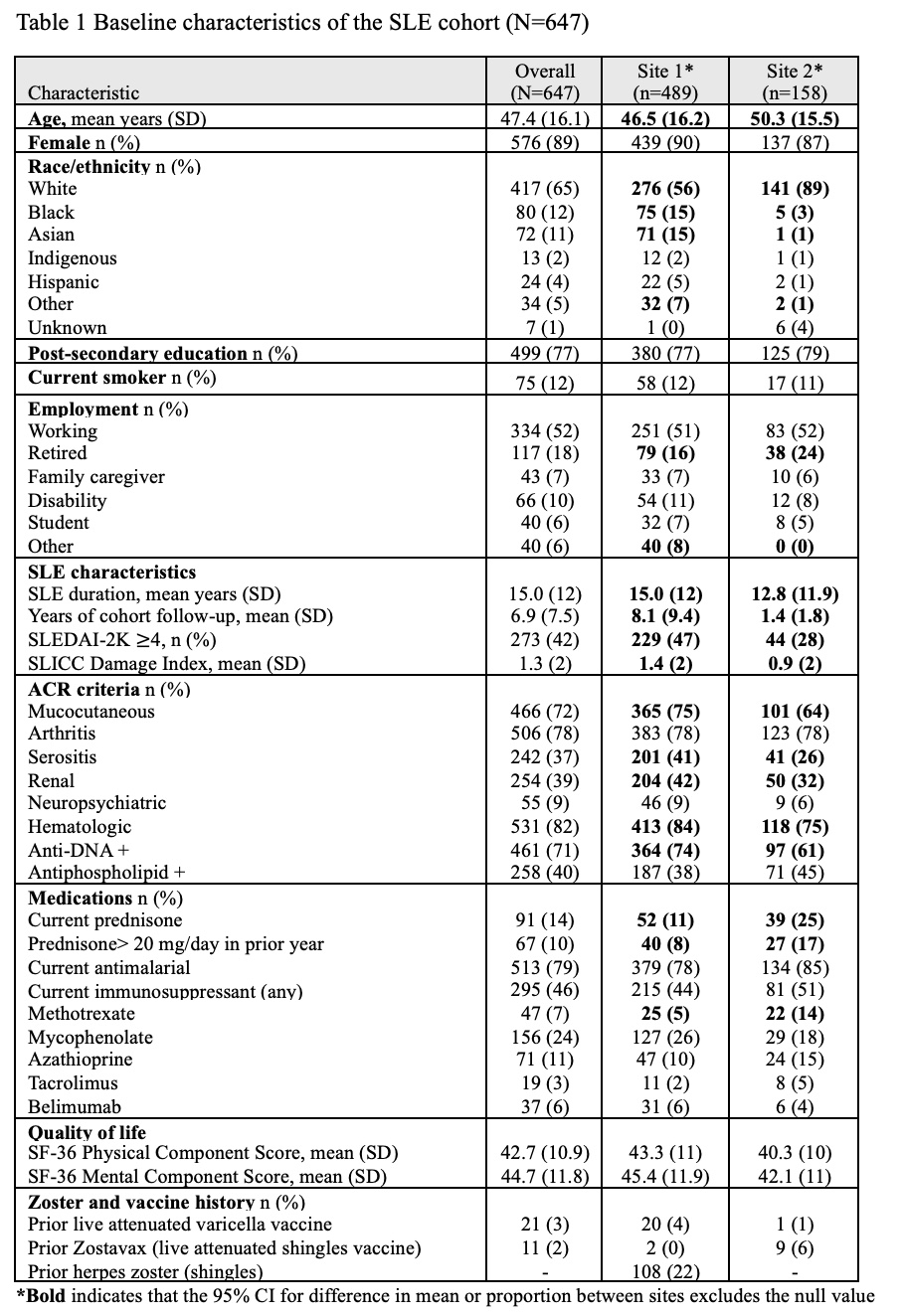
Results: Among 647 participants, 89% were female, 65% were White, and mean age was 47.4 years (SD 16), with an SLE duration of 15 years (SD 12). Nearly half (46%) were taking an immunosuppressant, primarily mycophenolate (24%). A history of previous shingles was reported in 108/489 (22%; data from one cohort only). Over the study follow-up, 172 (27%) received at least one RZV dose (Table 1). Vaccination rates were higher May 2023-December 2024 (16 per 100 person-years; 95% CI 13-20) compared to October 2017-April 2023 (3 per 100-person years; 95% CI 2-4). In multivariable analyses, age (each year, adjusted HR 1.04; 95% CI 1.02-1.05), White race/ethnicity (aHR 1.7; 95% CI 1.2-2.5), and immunosuppressant use (aHR 2.1, 95% 1.5-3.0) were associated with receiving the RZV, while prednisone use was potentially associated (HR 1.4, 95% CI 0.9-2.2). Among those who received a first RZV dose, 76/170 (45%) received the second dose within 3 months, and 120/139 (86%) received the second within 12 months (median time between doses 84 days, IQR 64-134).
Conclusion: Despite the young age of this cohort, nearly a quarter had previous shingles, highlighting a high burden in SLE. Although a provincial policy providing the 2-dose RZV free of charge to immunocompromised adults was associated with increased vaccination rates, less than a third of patients across both cohorts have been vaccinated. Interventions should target subgroups less likely to receive the vaccine (or complete the vaccination series).
To cite this abstract in AMA style:
Mendel A, Amiable N, St-Pierre Y, Bernatsky S, Colmegna I, Desjardins M, Grenier L, Kalache F, Pineau C, Sauvageau C, Vinet E, Fortin P. Increased Recombinant Zoster Vaccination in SLE following Public Reimbursement: Data from Two Prospective SLE Cohorts [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/increased-recombinant-zoster-vaccination-in-sle-following-public-reimbursement-data-from-two-prospective-sle-cohorts/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-recombinant-zoster-vaccination-in-sle-following-public-reimbursement-data-from-two-prospective-sle-cohorts/

.jpg)