Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Check point inhibitor PD-1 (programmed death protein 1) is upregulated during T lymphocyte activation and is important for limiting the duration of activation. Thus, maintain self-tolerance. Insufficient PD-1 signaling can lead to dysregulated T cell response. The functional significance of PD-1 and its ligands PD-L1/L2 is not studied in psoriatic arthritis (PsA). Our objectives were to (i) investigate the expression of PD-1 in PsA synovium (ii) determine its functional significance (iii) explore mechanistic of checkpoint agonists (Recombinant Human PD-L1/B7-H1; R&D system) as a novel therapy for T cells mediated autoimmune diseases.
Methods: Mononuclear cells from peripheral blood (PBMC) and synovial fluid (SFMC) of age/sex matched PsA patients (n=10), and RA patients (positive control, n=10) were isolated. Isolated CD3+ T cells (1x 106/ml) were activated with CD3/CD28 antibody (5mg each/ml) for 3 days in the presence/absence of recombinant PD-L1 (1µg/mL). Hi-D FACS studies were done : (1) to identify PD-1 in activated effector memory [CD4+/CD8+CD11a+CD45RO+] T (TEM) cells in PsA and RA SFMC; (2) to determine the anti-proliferative effect of recombinant PD-L1 on the SFMCs TEM cells by CFSE dilution test; (3) to determine the effect of recombinant PD-L1 agonist on the expression of IL-17A, IL-17F, IL-22 on SFMC TEM cells. Studies were performed using a FACSfusion Aria flow cytometer and data were analyzed using FlowJo software (Treestar, Ashland, OR). Western blot assays were done on lysates of the cultured CD3+T cells with recombinant PD-L1 in PsA and RA PBMC to determine effect on T cell signaling proteins (stat3/p-stat3, RORγt).
Results: High PD-1 expression (Fig 1) was observed in CD4+ (43.3%±12.6) and CD8+ (20.5%±6.5) activated TEM cells of PsA PBMC/SFMC and RA PBMC/SFMC. CFSE dilution assay showed proliferation of CD3/CD28 antibody stimulated memory CD4+ SFMC of PsA reduced significantly from 72.2%±2.2 to 30.1%±3.13 when treated with recombinant PD-L1 (p< 0.001). Similarly in RA SFMC with recombinant PD-L1, CD4+ TEM cell proliferation significantly reduced from 53.3%±2.6 to 26.2%±2.7 (p< 0.001).In CD3/CD28 stimulated PsA SFMC, Hi-D FACS analysis showed expression of IL-17A (9.2+0.8%), IL-17F (4.9+0.5%), and IL-22 (5.3+0.5%) in CD4+CD45RO+ T (TEM) cells were significantly reduced when treated with recombinant PD-L1 (4.3+0.8%, 4.9+0.5%, and 5.3+0.5%, respectively, p< 0.001). (Fig 2) Findings from western blot assays showed PD-L1 agonist treated PsA PBMC and RA PBMC activated CD3+ T cells had decreased expression of phospho-STAT3 compared to PD-L1 agonist untreated CD3+ T cells by 5-fold and 3-fold decrease, respectively (p< 0.001, Fig 3). Similar results were also seen in the expression of RORγt, the signaling proteins induced by rIL-23. (Fig 3)
Conclusion: Here we identified significance of PD-1 in PsA: (i) PD-1+TEM cells in SFMC of PsA are functional and regulates Th17 cell function through STAT3/ RORγt; (ii) recombinant human PD-L1 agonist significantly inhibited SFMC TEM cell proliferation and Th17 cell cytokine production (IL-17A, IL-17F, IL-22). Agonistic immune checkpoint-based therapy is likely to offer new avenues for treatment of psoriatic disease.
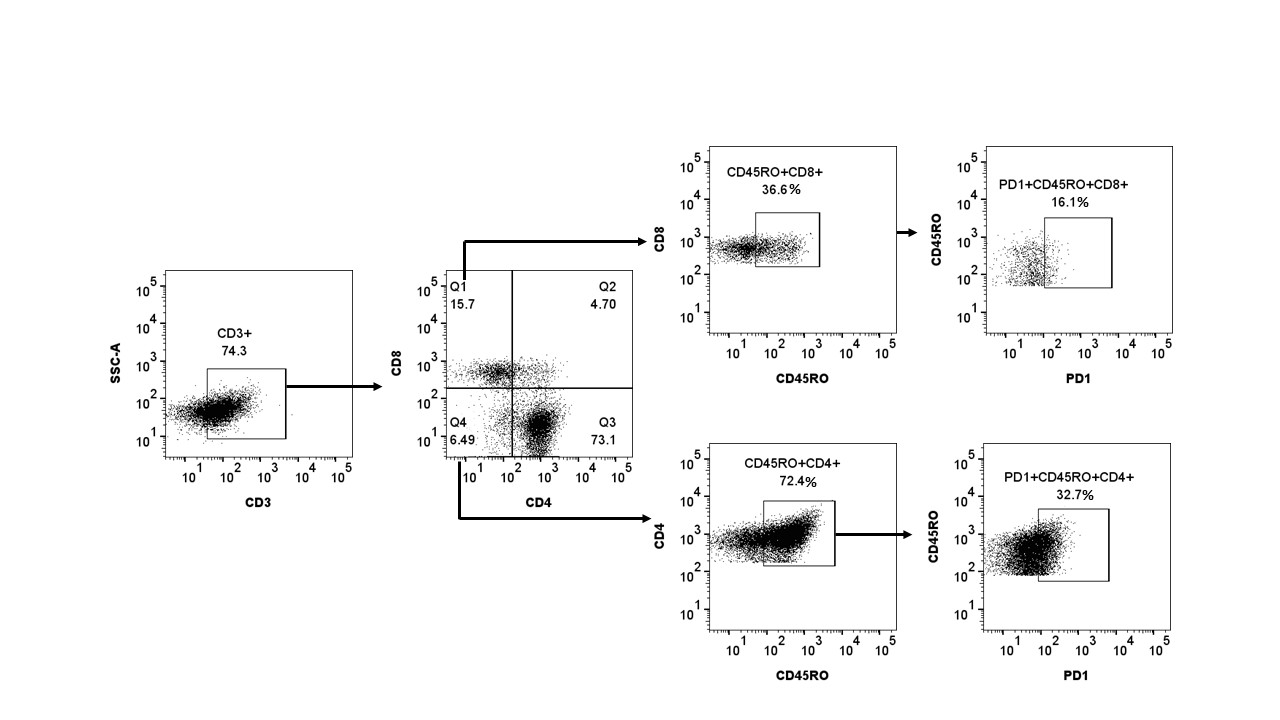 Figure 1. In flow cytometric Hi-D FACS study it was observed that a high number of psoriatic arthritis synovial CD4+/CD8+ Effector Memory T cells expressed Check point inhibitor PD-1 (programmed death protein 1). First activated live lymphomononuclear cells were gated and then effector memory [CD4+/CD8+CD11a+CD45RO+] T (TEM) cells were characterized.
Figure 1. In flow cytometric Hi-D FACS study it was observed that a high number of psoriatic arthritis synovial CD4+/CD8+ Effector Memory T cells expressed Check point inhibitor PD-1 (programmed death protein 1). First activated live lymphomononuclear cells were gated and then effector memory [CD4+/CD8+CD11a+CD45RO+] T (TEM) cells were characterized.
.jpg) Figure 2. Hi-D FACS study to determine the effect of recombinant PD-L1 agonist on the key pathologic Th17 cytokines (IL-17A, IL-17F, IL-22) which drives the pannus formation in psoriatic arthritis (PsA). Expression of IL-17A, IL-17F, and IL-22 in CD4+CD45RO+ T (TEM) cells were significantly reduced when CD3/CD28 stimulated PsA synovial fluid mononuclear cells were treated with recombinant human PD-L1 (1µg/mL).
Figure 2. Hi-D FACS study to determine the effect of recombinant PD-L1 agonist on the key pathologic Th17 cytokines (IL-17A, IL-17F, IL-22) which drives the pannus formation in psoriatic arthritis (PsA). Expression of IL-17A, IL-17F, and IL-22 in CD4+CD45RO+ T (TEM) cells were significantly reduced when CD3/CD28 stimulated PsA synovial fluid mononuclear cells were treated with recombinant human PD-L1 (1µg/mL).
.jpg) Figure 3. PD-1 activation in T cells regulates Th17 cell function by inhibiting IL-23 induced STAT3/ RORγt activation. Western blot assays were done on lysates of the cultured CD3+T cells. PBMC T cells from psoriatic arthritis (PsA) and rheumatoid arthritis (RA) were stimulated with anti-human CD3 (1µg/mL) and CD28 (1µg/mL) and rIL-23 (40ng/mL) in presence/absence of recombinant human PD-L1 agonist (1µg/mL). AS expected rIL-23 induced phosphorylation of STAT3 and upregulated RORγt expression. PD-L1 agonist treated PsA PBMC and RA PBMC CD3+ T cells had significant decreased expression of phospho-STAT3 compared to untreated CD3+ T cells (p < 0.001). Similar effect was seen on the expression of RORγt.
Figure 3. PD-1 activation in T cells regulates Th17 cell function by inhibiting IL-23 induced STAT3/ RORγt activation. Western blot assays were done on lysates of the cultured CD3+T cells. PBMC T cells from psoriatic arthritis (PsA) and rheumatoid arthritis (RA) were stimulated with anti-human CD3 (1µg/mL) and CD28 (1µg/mL) and rIL-23 (40ng/mL) in presence/absence of recombinant human PD-L1 agonist (1µg/mL). AS expected rIL-23 induced phosphorylation of STAT3 and upregulated RORγt expression. PD-L1 agonist treated PsA PBMC and RA PBMC CD3+ T cells had significant decreased expression of phospho-STAT3 compared to untreated CD3+ T cells (p < 0.001). Similar effect was seen on the expression of RORγt.
To cite this abstract in AMA style:
Raychaudhuri S, Abria C, Raychaudhuri S. Immune Checkpoint agonists: A New horizon for treatment of psoriatic arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/immune-checkpoint-agonists-a-new-horizon-for-treatment-of-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-checkpoint-agonists-a-new-horizon-for-treatment-of-psoriatic-arthritis/
