Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite the good efficacy of bDMARDs in nearly 60% of patients with axial spondyloarthritis (AxSpA), high costs limit their use in resource-limited settings. Few studies suggest JAK inhibitors to be more accessible and cost-effective alternative
Methods: Objective: This study aimed to evaluate the long-term effectiveness and safety of generic tofacitinib in Indian patients with active axial SpA.Materials and Methods:This retrospective single-center study included 290 patients aged ≥18 years with active axial spondyloarthritis (axSpA), defined by BASDAI ≥4 or ASDAS ≥2.1, who were initiated on generic tofacitinib between January 2021 and March 2023, despite prior treatment with csDMARDs and/or bDMARDs. Only patients with a minimum follow-up of one year were included. Baseline data—including age, sex, disease duration, comorbidities, smoking status, HLA-B27 status, inflammatory markers (ESR, CRP), presence of uveitis, inflammatory bowel disease, enthesitis, disease activity indices (BASDAI, ASDAS), and concomitant medications—were recorded. Follow-up data on disease activity was systematically extracted from prospectively maintained electronic medical records. The primary endpoint was the proportion of patients achieving inactive disease or low disease activity (ASDAS < 2.1). Failure to meet this target within 12–24 weeks, or later in the event of disease flares, was classified as treatment failure. The details of adverse events at follow up visits were also noted
Results: Baseline characteristics of 290 patients included in the study are summarized in Table 1. After a median follow-up of 25 months (IQR 15-33), 258 of 290 patients (89%) achieved either inactive disease with ASDAS < 1.3 (n=112, 39%) or Low disease activity defined by ASDAS 1.3–2.1(n=146,50%) [Figure 1]. Tofacitinib failed in 32 patients (11%) of whom 10 (3.4%) patients had initial response but flared later. No significant predictors of non-response were identified.Adverse events were reported in 52 patients (17.9%), most commonly transaminitis (42, 14.5%), followed by dyslipidemia (5, 1.7%), herpes zoster (3, 1%), and tuberculosis (2, 0.7%). Ten (3.4%) patients discontinued tofacitinib due to adverse effects. Transaminitis occurred at a median of 8 months and was associated with fatty liver in 29 patients (10%). Most cases of transaminitis (37, 12.7%) were mild and resolved with dose reduction. No serious infections, major adverse cardiovascular events (MACE), thromboembolic events, or deaths were observed.
Conclusion: Tofacitinib was effective and well-tolerated for controlling disease activity in active axial SpA in real life settings
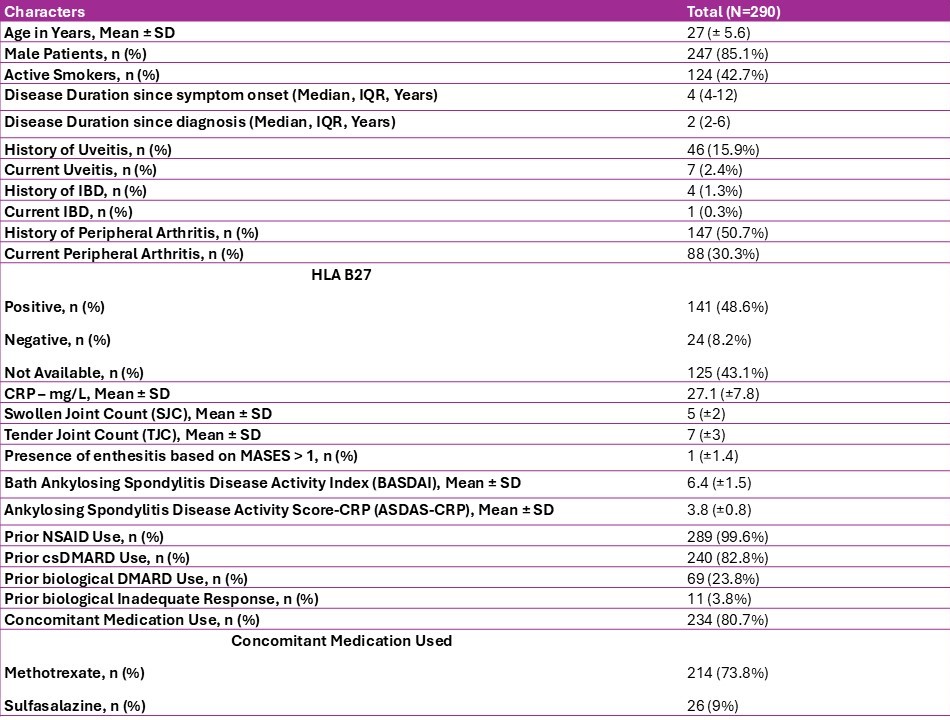 Table-1: Baseline characteristics of study population
Table-1: Baseline characteristics of study population
.jpg) Figure-1: Sankey diagram depicting the efficacy of tofacitinib in patients with active axial SpA
Figure-1: Sankey diagram depicting the efficacy of tofacitinib in patients with active axial SpA
To cite this abstract in AMA style:
Vasantha Kumar P, Mathew J, Jacob Mathew A, Goel R. Long-Term Efficacy and Safety of Tofacitinib in Active Axial Spondyloarthritis: Experience from a Tertiary Care Center in South India [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-tofacitinib-in-active-axial-spondyloarthritis-experience-from-a-tertiary-care-center-in-south-india/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-tofacitinib-in-active-axial-spondyloarthritis-experience-from-a-tertiary-care-center-in-south-india/
