Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The prevalence of cardiometabolic diseases, including obesity and diabetes, is higher in patients with psoriatic arthritis (PsA) than those without PsA.1,2 Metabolic syndrome is a common comorbidity of PsA that can impact treatment choice.3 Weight loss and improvements in other cardiometabolic biomarkers have been observed with apremilast (APR) treatment in randomized controlled trials and real-world studies.4-6 The aim of this post hoc analysis was to evaluate the effects of APR on body mass index (BMI), weight, and HbA1c over 48 weeks in patients with early oligoarticular (oligo) PsA from the FOREMOST trial (NCT03747939).
Methods: FOREMOST was a phase 4, multicenter, randomized, double-blind, placebo (PBO)-controlled trial that enrolled patients with early (duration ≤5 years) oligo PsA ( >1 to ≤4 swollen joints and >1 to ≤4 tender joints; 66–68 joints assessed). Patients were randomized 2:1 to APR (n=203) or PBO (n=105) for 24 weeks (early escape at Week 16), followed by an extension phase in which all patients received APR through Week 48. Percent changes from baseline to Weeks 16 and 48 in BMI and weight were assessed. The percent change in weight from baseline to Week 48 was assessed overall and by baseline BMI category (normal: < 25 kg/m2, overweight: ≥25 to < 30 kg/m2, class I–II obesity: ≥30 to < 40 kg/m2, class III obesity: ≥40 kg/m2). Additionally, changes in BMI and HbA1c category (normal: < 5.7%, pre-diabetic: ≥5.7% to < 6.5%, and diabetic: ≥6.5%) were evaluated from baseline to Week 48.
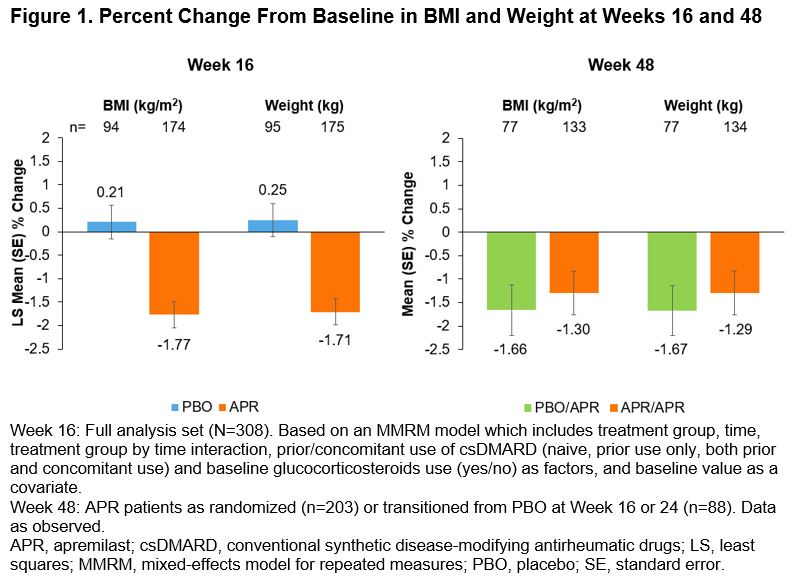
Results: Baseline BMI, weight, and HbA1c were similar between APR and PBO patients (30.2 vs 31.4 kg/m2, 87.3 vs 91.5 kg, and 5.8 vs 5.7%, respectively). Greater percent reductions in BMI (-1.77 vs 0.21%) and weight (-1.71 vs 0.25%) were seen with APR vs PBO at Week 16 (Figure 1). Decreases in BMI and weight were maintained through Week 48 with continued APR treatment (APR/APR; Figure 1); similar improvements were seen in patients who transitioned to APR at Week 24 (PBO/APR). Over the 48-week period, a greater proportion of patients randomized to APR experienced weight loss than weight gain. More than 50% of patients had a weight reduction of ≥1% and approximately 20% achieved a reduction of ≥5% (Figure 2). Among patients randomized to APR, there was a general shift in BMI and HbA1c categories from baseline toward lower-risk categories after 48 weeks of treatment (Figure 3).
Conclusion: APR treatment through Week 48 was associated with improvements in BMI and weight in the FOREMOST study of early oligo PsA. Consistent with prior studies of APR in polyarticular PsA and psoriasis, greater changes in cardiometabolic markers were observed in patients in the highest risk categories (ie, obesity and diabetic subgroups). References1. Kumthekar A, Ogdie A. Rheumatol Ther. 2020;7(3):447–456.2. Dubreuil M et al. Rheumatology (Oxford). 2014;53(2):346–352. 3. Coates LC et al. Nat Rev Rheumatol. 2022;18(8):465–479.4. Cavanaugh C et al. JAAD Int. 2024;16:244–251. 5. Gelfand JM et al. JAMA Dermatol. 2022;158(12):1394–1403.6. Mease PJ, et al. Presented at: European Alliance of Associations for Rheumatology Annual Meeting; May 31–June 3, 2023; Milan, Italy.
To cite this abstract in AMA style:
Mease P, González Cantero Á, Soung J, Armstrong A, Merola J, Proft F, Gossec L, Gladman D, Coates L, Teng L, Vázquez J, Deignan C, Kavanaugh A. Effects of Apremilast on Body Mass Index, Weight, and HbA1c as Cardiometabolic Outcomes in Patients With Early Oligoarticular Psoriatic Arthritis in the FOREMOST Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effects-of-apremilast-on-body-mass-index-weight-and-hba1c-as-cardiometabolic-outcomes-in-patients-with-early-oligoarticular-psoriatic-arthritis-in-the-foremost-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-apremilast-on-body-mass-index-weight-and-hba1c-as-cardiometabolic-outcomes-in-patients-with-early-oligoarticular-psoriatic-arthritis-in-the-foremost-study/

.jpg)
.jpg)