Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: FOREMOST (NCT03747939)1 offers novel insights on early oligoarticular (oligo, ≤4 active joints) PsA. Our aim was to analyse progression to polyarticular (poly; >4 active joints) disease and predictors of progression in FOREMOST.
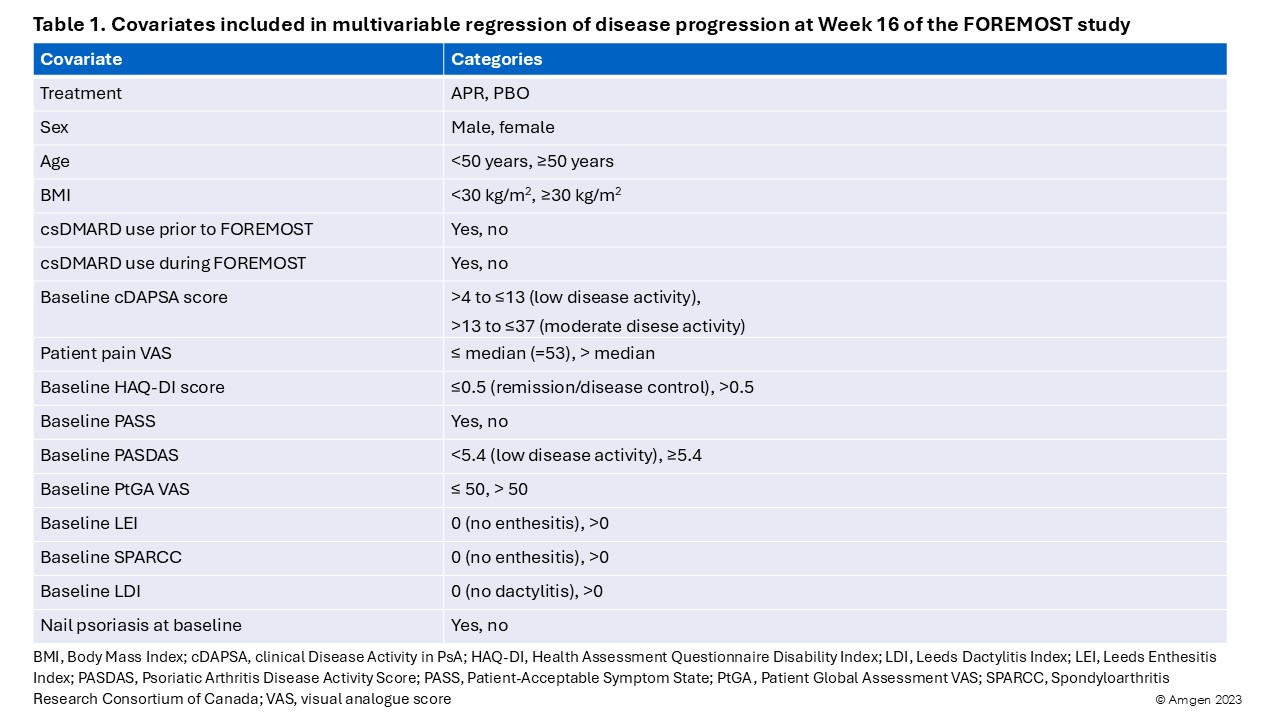
Methods: FOREMOST is a randomized trial of 308 patients with early (duration ≤5 years) oligo PsA and limited joint involvement ( >1 to ≤4 swollen and >1 to ≤4 tender joints; 66–68 joints assessed) who received apremilast (APR) (n=203) or placebo (PBO) (n=105) for 24 weeks, with a primary outcome at 16 weeks1. Standard of care (SoC) NSAIDs and/or conventional synthetic (cs) DMARDs were permitted if taken at a stable dose through Week 24. Progression to poly disease was defined as changing from ≤4 active (swollen and/or tender) joints at baseline to >4 active joints at Week 16. In a post hoc, multivariable regression analysis, we assessed predictors of progression in the overall FOREMOST population and the placebo (PBO)±SOC group (as a surrogate for natural history). Variables of interest were selected on clinical relevance (Table 1) and backward selection used to identify statistically significant predictors of progression (p< 0.05 threshold); based on clinical relevance, body mass index (BMI) and enthesitis (Leeds index, LDI) were forced to remain in the model. Missing joint data were imputed using last observation carried forward.
Results: In FOREMOST, most patients had ≤4 active joints at baseline (overall, 268/308 [87%]; APR, 176/203 [87%]; PBO±SOC, 92/105 [88%]) and baseline characteristics were similar between APR and PBO±SOC1. In the overall population, APR was associated with statistically significant lower odds of progression vs PBO±SOC (Odds Ratio [OR] [95% CI], 0.42 [0.22, 0.77]; Fig 2a; adjustment for other covariates). After adjusting for treatment, being female (vs male), not receiving (vs receiving) csDMARDs during the study, and enthesitis (SPARCC >0; vs no enthesitis) were associated with statistically significantly higher odds of progression; patients with dactylitis (LDI >0) showed a non-significant trend towards higher odds of progression vs patients without dactylitis. In the PBO±SOC group, being female, being csDMARD-naive (vs prior csDMARD use), dactylitis (LDI > 0; vs no dactylitis), and not having nail psoriasis (vs having nail psoriasis) were associated with statistically significant higher odds of progression (Fig 2b).
Conclusion: Our data provides novel insights into factors driving progression from oligo to poly PsA, with female sex, enthesitis and dactylitis found to increase the risk of progression. These data may help clinicians identify patients with poor prognosis who require close monitoring and more intensive treatment. Among patients in FOREMOST receiving PBO, prior csDMARD use decreased the likelihood of progression more than three-fold. Compared with PBO±SOC, APR more than halved the likelihood of progression. These findings highlight the importance of early intervention in oligo PsA and the benefit of APR in delaying/preventing progression to poly disease. Our results should be validated using other PsA cohorts.1. Gossec, L., et al. Ann Rheum Dis, 2024. 83(11): p. 1480-1488.
To cite this abstract in AMA style:
Coates L, Mease P, Merola J, Perez Chada L, Gladman D, Zabotti A, Mrowietz U, kishimoto m, Deignan C, Chaudhari S, Teng L, Gossec L. Factors impacting progression from oligoarticular to polyarticular PsA: Data from the FOREMOST study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/factors-impacting-progression-from-oligoarticular-to-polyarticular-psa-data-from-the-foremost-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-impacting-progression-from-oligoarticular-to-polyarticular-psa-data-from-the-foremost-study/

.jpg)