Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: A substantial number of patients with rheumatic inflammatory/autoimmune diseases, do not achieve adequate symptom control with current standard-of-care therapies, including biologics. The endocannabinoid system may offer an alternative therapeutic target. Recently we found a significant change in the circulating level of endogenous cannabinoids (endocannabinoids) in rheumatoid arthritis (RA) and fibromyalgia syndrome (FMS) patients as compared to healthy controls. The current study aimed to evaluate ex vivo anti-inflammatory effects of non-psychoactive phytocannabinoids and endocannabinoids in peripheral blood mononuclear cells (PBMCs) from patients with refractory rheumatic inflammatory/autoimmune conditions.
Methods: PBMCs were isolated from psoriatic arthritis (PsA) and ankylosing spondylitis (AS) patients who were unresponsive to anti-TNFα treatment, as well as from FMS patients. PBMCs were activated ex-vivo using PHA and LPS, and treated with either phytocannabinoids (CBD, CBC, CBG) or endocannabinoids (AEA, PEA, OEA). Cytokine levels (TNF-α, IFN-γ, IL-17, IL-6 and IL-1β) were measured in cell supernatants and plasma using ELISA assay.
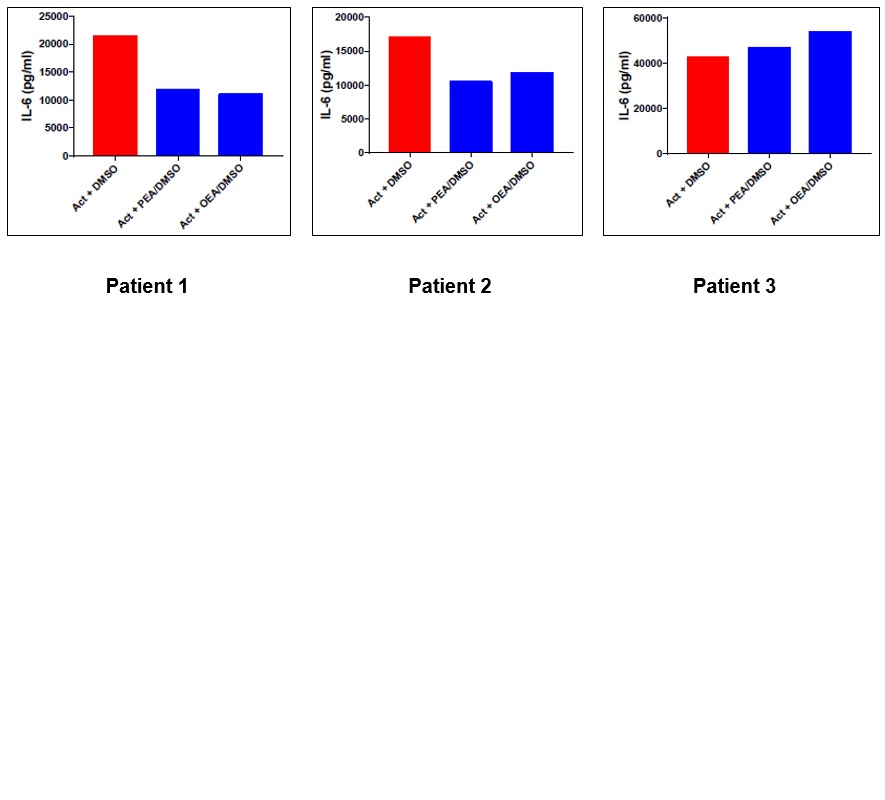
Results: In PBMCs from severe refractory PsA patient, the phytocannabinoid CBD demonstrated superior suppression of pro-inflammatory cytokines (IL-6, IL-17, and IFN-γ) compared to the corresponding anti-TNFα biologics while both CBD and anti-TNFα abrogate TNFα secretion (Fig. 1). In PBMCs from refractory AS patients (n=3), phytocannabinoids (CBC and CBG) demonstrated superior suppression of IFN-γ and IL-6 compared to the corresponding anti-TNFα biologics (Fig. 2). In FMS patients (n=3), endocannabinoids (AEA, PEA, and OEA) modulated IL-6 secretion in a patient-specific manner, with variable cytokine responses observed (Fig. 3).
Conclusion: Non-psychoactive phytocannabinoids exhibit robust ex vivo anti-inflammatory activity in PBMCs from refractory PsA and AS patients, surpassing anti-TNFα biologics. Additionally, individualized endocannabinoid responses in FMS support a precision medicine approach. These findings highlight the potential of integrating phytocannabinoids and endocannabinoid profiling to guide personalized therapeutic strategies in rheumatic inflammatory/autoimmune diseases.
 The cannabinoid CBD reduce the level of secreted IL-6, IFN-γ and IL-17 from activated PBMC of refractory severe PsA patient, much better than Anti-TNFα mAb (Remicade®) therapy. Human PBMCs were isolated from severe (refractory to Anti-TNFα therapy) PsA patient (DAS-28: 6.49), seeded (500,000 cells/0.5ml/well) and activated using LPS (100ng/ml) + PHA (10µg/ml) for 24h. Anti-TNFα mAb (Remicade®) or CBD (1/3 less concentrated than Remicade) were added concomitantly to activation, for a total of 24h. TNFα, IL-6, IL-17A and IFNγ cytokine levels were measured in the supernatants, 24 h after activation, by ELISA assay. Act – Activation using LPS/PHA.
The cannabinoid CBD reduce the level of secreted IL-6, IFN-γ and IL-17 from activated PBMC of refractory severe PsA patient, much better than Anti-TNFα mAb (Remicade®) therapy. Human PBMCs were isolated from severe (refractory to Anti-TNFα therapy) PsA patient (DAS-28: 6.49), seeded (500,000 cells/0.5ml/well) and activated using LPS (100ng/ml) + PHA (10µg/ml) for 24h. Anti-TNFα mAb (Remicade®) or CBD (1/3 less concentrated than Remicade) were added concomitantly to activation, for a total of 24h. TNFα, IL-6, IL-17A and IFNγ cytokine levels were measured in the supernatants, 24 h after activation, by ELISA assay. Act – Activation using LPS/PHA.
.jpg) The cannabinoids (CBG and CBC) show superior reduction in the level of secreted IFNγ and IL-6 from activated PBMCs of refractory ankylosing spondylitis (AS) patients (n=3) vs. the non-effective Anti-TNFα mAb (Remicade®). Human PBMCs were isolated from severe (refractory to Anti-TNFα therapy) AS patient, seeded (500,000 cells/0.5ml/well) and activated using LPS (100ng/ml) + PHA (10µg/ml) for 24h. Anti-TNFα mAb (Remicade®) or CBC/CBG (1/3 less concentrated than Remicade) were added concomitantly to activation, for a total of 24h. IFNγ and IL-6 cytokine levels were measured in the supernatants, 24 h after activation, by ELISA assay. Act – Activation using LPS/PHA.
The cannabinoids (CBG and CBC) show superior reduction in the level of secreted IFNγ and IL-6 from activated PBMCs of refractory ankylosing spondylitis (AS) patients (n=3) vs. the non-effective Anti-TNFα mAb (Remicade®). Human PBMCs were isolated from severe (refractory to Anti-TNFα therapy) AS patient, seeded (500,000 cells/0.5ml/well) and activated using LPS (100ng/ml) + PHA (10µg/ml) for 24h. Anti-TNFα mAb (Remicade®) or CBC/CBG (1/3 less concentrated than Remicade) were added concomitantly to activation, for a total of 24h. IFNγ and IL-6 cytokine levels were measured in the supernatants, 24 h after activation, by ELISA assay. Act – Activation using LPS/PHA.
.jpg) The effect of N-arachidonoylethanolamine (AEA) on IL-6 secretion from LPS/PHA-induced activated PBMCs of FMS patients. Human PBMCs were isolated from FMS patients, seeded (500,000 cells/0.5ml/well) and activated using LPS (100ng/ml) + PHA (10µg/ml) for 24h. AEA (10^-6M) was added 5h after the intial activation, for a total of 24h. IL-6 cytokine level was measured in the supernatants, 24 h after activation, by ELISA assay. Act – Activation using LPS/PHA; Eth – Ethanol which used to dissolve the AEA.
The effect of N-arachidonoylethanolamine (AEA) on IL-6 secretion from LPS/PHA-induced activated PBMCs of FMS patients. Human PBMCs were isolated from FMS patients, seeded (500,000 cells/0.5ml/well) and activated using LPS (100ng/ml) + PHA (10µg/ml) for 24h. AEA (10^-6M) was added 5h after the intial activation, for a total of 24h. IL-6 cytokine level was measured in the supernatants, 24 h after activation, by ELISA assay. Act – Activation using LPS/PHA; Eth – Ethanol which used to dissolve the AEA.
To cite this abstract in AMA style:
Halpert G, Govrin E, Gendelman O, Watad A, Shoenfeld Y, Amital H. Phyto- and Endo-cannabinoids Exhibit Ex Vivo Anti-Inflammatory Effects in Refractory Rheumatic inflammatory/Autoimmune Patients: Toward Personalized Cannabinoid Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/phyto-and-endo-cannabinoids-exhibit-ex-vivo-anti-inflammatory-effects-in-refractory-rheumatic-inflammatory-autoimmune-patients-toward-personalized-cannabinoid-therapy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phyto-and-endo-cannabinoids-exhibit-ex-vivo-anti-inflammatory-effects-in-refractory-rheumatic-inflammatory-autoimmune-patients-toward-personalized-cannabinoid-therapy/
