Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: PsA affects ~20-30% of pts with PsO, causing articular inflammation/damage, and impaired health-related quality of life (HRQoL). ICO, a novel targeted oral peptide that binds IL-23 Receptor, showed greater clinical response rates vs placebo (PBO) and a favorable safety profile in pts with moderate-to-severe plaque PsO in the Ph 2 FRONTIER 1&2 studies. We leveraged exploratory serum biomarker, Pt Reported Outcomes Measurement Information Systems (PROMIS-29), and Psoriasis Area and Severity Index 75% improvement (PASI75) data from a subset of FRONTIER 1 pts with PsO and PsA medical history (PsO+PsA) to support Ph 3 ICONIC-PsA 1 and ICONIC-PsA 2 studies.
Methods: Mean log fold-changes (logFC) in serum β-Defensin-2 (BD-2), IL-22, IL-17A, and IL-17F levels were summarized for FRONTIER pts with PsO only, and with PsO+PsA. Pts reported HRQoL via PROMIS-29 questionnaire. Improvements ≥5-points in PROMIS-29 domain scores (or ≥2 for pain), and physical/mental component summary (PCS/MCS) scores, are considered clinically meaningful. ICO PsA Ph 3 sample sizes were informed by ample safety data to support regulatory submission and estimates from model-based analyses, including a meta-analysis that bridged FRONTIER 1 PASI75 to expected American College of Rheumatology 20% improvement (ACR20) at Week (W)16 (primary endpoint) and meta-regression modeling that bridged ACR20 to secondary endpoints, ACR50/70, PASI90/100, Minimal Disease Activity.
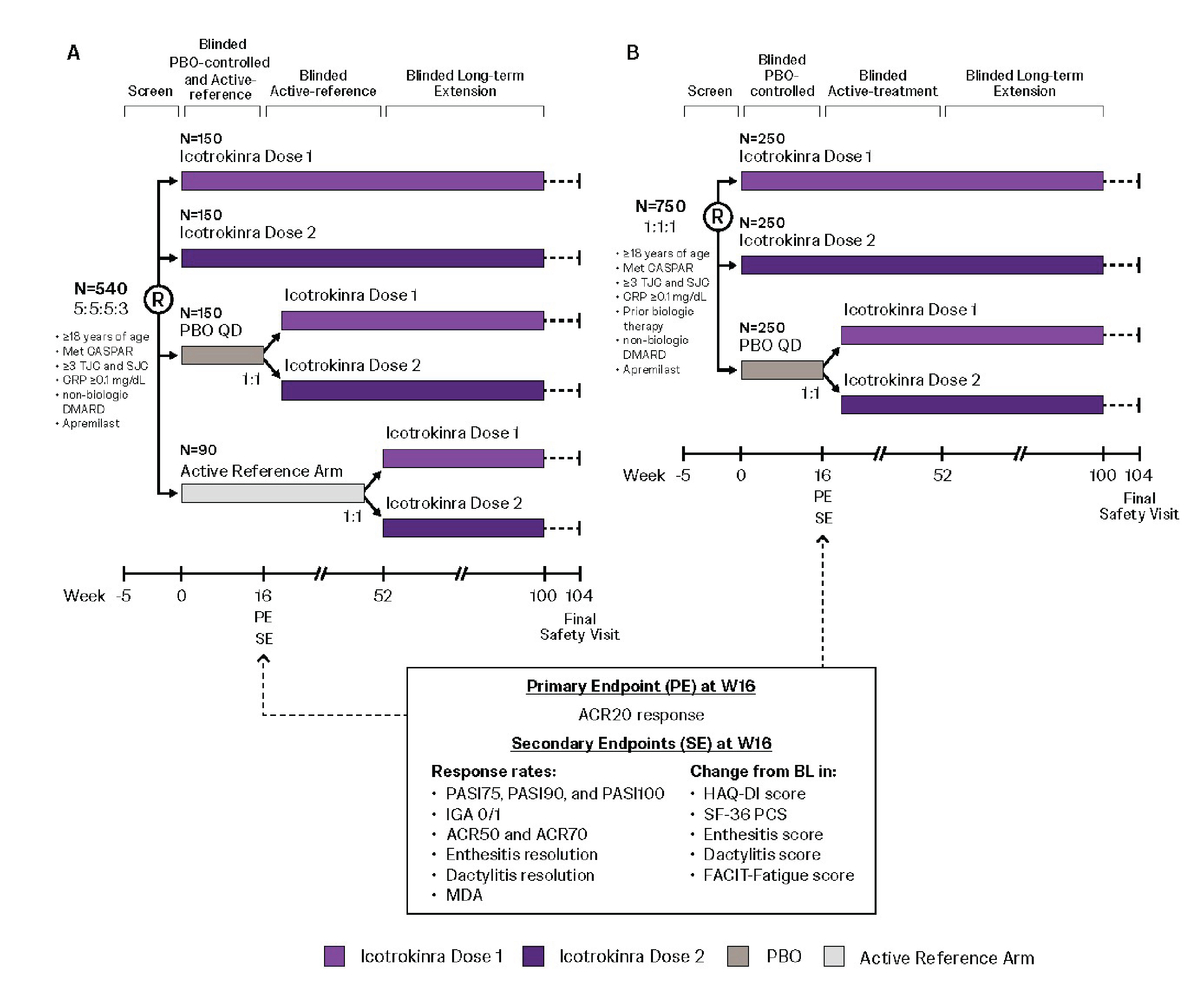
Results: 23/91 (25%) FRONTIER 1 pts had PsO+PsA (ICO, n=20; PBO, n=3). Among pts with evaluable biomarker data, mean logFC in serum BD-2, IL-22, IL-17A (Fig 1A-C), and IL-17F (data not shown) over time indicated consistent ICO pharmacodynamic (PD) effect between pts with PsO only and with PsO+PsA. ICO-treated PsO+PsA pts reported numerically greater mean changes from baseline (BL) at W16 vs PBO across PsA relevant PROMIS-29 domains, ie, improvements in physical function (7.7 vs 1) and reductions in fatigue (-7.4 vs 2.3 [worsening]), pain interference (-10.7 vs -1.3), and pain intensity (- 3.5 vs -1.5). In 20 ICO-treated pts with PsO+PsA, 45% and 70% reported ≥5 point improvement from BL in PROMIS-29 PCS and MCS scores, respectively, vs no pts receiving PBO. ICONIC-PsA 1&2 sample sizes of 540 (5:5:5:3 to ICO Dose 1/2/PBO/active reference arm with no formal statistical comparisons; Fig 2A) and 750 (1:1:1 to ICO Dose 1/2/PBO; Fig 2B) were estimated to provide ≥90% power to detect significant ICO vs PBO difference between ICO and PBO. Given minorities are often under-recruited in PsA trials, the ICO PsA program aims to assess diverse pts, of different racial/ethnicities.
Conclusion: Exploratory assessments from the Ph 2 FRONTIER 1 study showed comparable ICO PD effects between pts with PsO only, and with PsO+PsA. ICO-treated pts with PsO+PsA reported numerically greater improvements in PsA-relevant PROMIS-29 domains vs PBO. Informed by these post hoc analyses and model-based meta-analyses that bridged data from PsO to PsA, the multicenter, double-blind, PBO-controlled ICONIC-PsA 1 and ICONIC-PsA 2 studies will comprehensively evaluate the novel targeted oral peptide ICO in diverse population of pts with active PsA.
 Mean change in serum BD-2 (A), IL-22 (B), and IL-17A (C) levels through W16 in FRONTIER 1 participants with PsO only and PsO+PsA
Mean change in serum BD-2 (A), IL-22 (B), and IL-17A (C) levels through W16 in FRONTIER 1 participants with PsO only and PsO+PsA
.jpg) ICONIC PsA-1 (biologic-naïve [A]) and ICONIC PsA-2 (biologic-experienced [B]) study designs
ICONIC PsA-1 (biologic-naïve [A]) and ICONIC PsA-2 (biologic-experienced [B]) study designs
To cite this abstract in AMA style:
Merola J, Mease P, Coates L, McInnes I, Nash P, Ogdie A, Eder L, kishimoto m, Beutler A, Psachoulia K, Sheng S, Zhou B, Javidi M, Valiathan C, Iaconangelo C, Yang Y, Kannan A, Karyekar C, Shagroni T. Icotrokinra (ICO), a Novel Targeted Oral Peptide, in Patients (Pts) With Psoriatic Disease: Exploratory Assessments From a Phase 2 Psoriasis (PsO) Study Informing a Phase 3 Clinical Program in Psoriatic Arthritis (PsA) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/icotrokinra-ico-a-novel-targeted-oral-peptide-in-patients-pts-with-psoriatic-disease-exploratory-assessments-from-a-phase-2-psoriasis-pso-study-informing-a-phase-3-clinical-program-in-psoriat/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/icotrokinra-ico-a-novel-targeted-oral-peptide-in-patients-pts-with-psoriatic-disease-exploratory-assessments-from-a-phase-2-psoriasis-pso-study-informing-a-phase-3-clinical-program-in-psoriat/
