Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Axial spondyloarthritis (AxSpA) is an inflammatory condition that falls under the category of spondyloarthritis (SpA) and significantly affects quality of life, causing symptoms like fatigue, sleep disturbances, depression, and sexual dysfunction, which can greatly diminish health-related quality of life and restrict work, leisure activities, and daily living. This underscores the urgent need for effective treatment strategies. Tofacitinib, a Janus kinase inhibitor, is used for various inflammatory conditions, but there is limited real-world evidence on its efficacy and safety for AxSpA in Indian patients. This retrospective study aimed to evaluate the effectiveness and safety of tofacitinib in Indian patients diagnosed with AxSpA in a real-world setting.
Methods: A single-centre, retrospective, observational study was conducted on patients with active AxSpA in India, spanning from November 2023 to April 2024. The clinical and laboratory records of AxSpA patients who were treated with generic tofacitinib at a dosage of 5 mg twice daily for 24 weeks were reviewed and analysed. The efficacy of tofacitinib was assessed based on the improvement in disease activity, specifically the change in the Ankylosing Spondylitis Disease Activity Score C-Reactive Protein (ASDAS-CRP) after 24 weeks. Adverse events (AEs) were monitored throughout the treatment period.
Results: A total of 100 patients with active AxSpA participated in the study, with a mean age of 35.95 years and an average disease duration of 8.20 years. Of these, 77 were males and 23 were females. All patients received tofacitinib 5 mg twice daily for 24 weeks. The majority of the patients were biologic-naive, with only 12 having prior biologic therapy. Additionally, 23 patients received tofacitinib monotherapy without nonsteroidal anti-inflammatory drugs (NSAIDs), while 77 patients received tofacitinib along with on-demand NSAIDs but without any concomitant conventional disease-modifying antirheumatic drug therapy. It showed a significant decrease in the ASDAS-CRP score from 2.94 to 1.18 by week 24, with a mean difference of 1.76 (95% confidence interval [CI]: 1.56-1.96, P < 0.001). The CRP levels also dropped significantly, from 13.85 mg/L to 4.69 mg/L at week 24, with a mean difference of 9.16 (95% CI: 7.23-11.09, P < 0.001). Notably, nearly 71% of patients achieved inactive disease status (ASDAS-CRP < 1.3) after 24 weeks of treatment, and tofacitinib was well tolerated in this cohort, with a low incidence of AEs like non-serious herpes zoster (6.00%), respiratory system-related infections (3.00%), and pulmonary thrombo-embolism (1.00%).
Conclusion: This retrospective real-world study supports the efficacy and safety of generic tofacitinib as an NSAID-sparing agent for Indian patients with active AxSpA. Its cost-effectiveness compared to expensive biologics and the convenience of oral administration make it an appealing option, especially in areas with limited access to biologics. Tofacitinib could improve patient outcomes and provide an affordable therapy option for managing AxSpA.
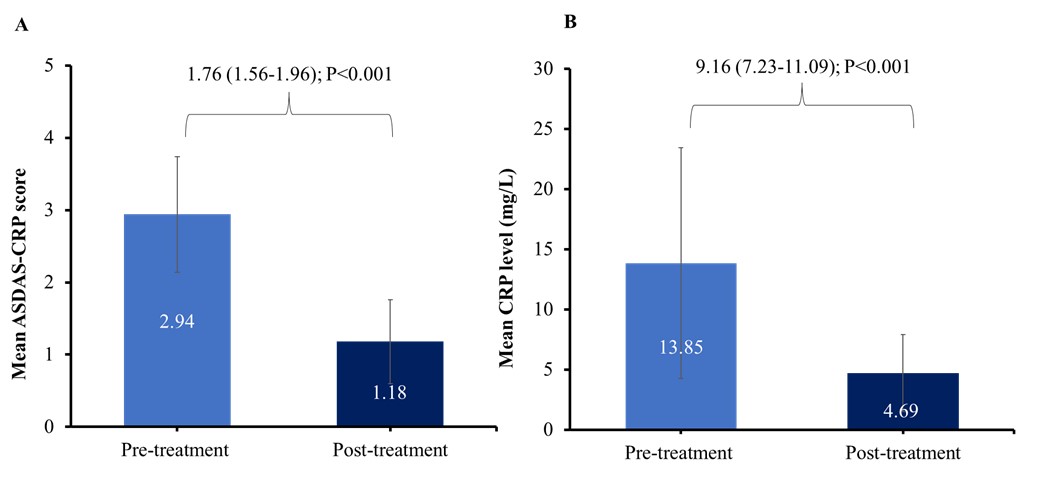 Figure 1: A) Mean ASDAS-CRP scores and B) Mean CRP level at pre-treatment and post-treatment with tofacitinib at 24 weeks (Nf100)
Figure 1: A) Mean ASDAS-CRP scores and B) Mean CRP level at pre-treatment and post-treatment with tofacitinib at 24 weeks (Nf100)
.jpg) Figure 2: Disease activity at baseline and 24 weeks (post-treatment with tofacitinib) in AxSpA patients (ASDAS-CRP, axial spondyloarthritis disease activity score C-reactive protein; CRP, C-reactive protein)
Figure 2: Disease activity at baseline and 24 weeks (post-treatment with tofacitinib) in AxSpA patients (ASDAS-CRP, axial spondyloarthritis disease activity score C-reactive protein; CRP, C-reactive protein)
.jpg) Table 1: Baseline characteristics
Table 1: Baseline characteristics
To cite this abstract in AMA style:
Kapoor S, Bondage B, Kedia N, Patel M, Kapoor A. Real world experience with generic Tofacitinib in Axial Spondyloarthritis from North India [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-experience-with-generic-tofacitinib-in-axial-spondyloarthritis-from-north-india/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-experience-with-generic-tofacitinib-in-axial-spondyloarthritis-from-north-india/
