Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: Therapies (0873–0878)
Session Type: Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: Icotrokinra (ICO), a novel targeted oral peptide, binds the interleukin (IL)-23 receptor to inhibit IL-23 signaling. ICONIC-LEAD (NCT06095115) is the first Phase 3 study in moderate-to-severe psoriasis to assess the efficacy and safety of a systemic therapy simultaneously in adolescents and adults.
Methods: In ICONIC-LEAD, adolescents (12 to < 18 years) with moderate-to-severe psoriasis (body surface area [BSA] ≥10%, Psoriasis Area and Severity Index [PASI] ≥12, Investigator’s Global Assessment [IGA] ≥3) and body weight ≥40 kg were randomized 2:1 to once-daily ICO 200 mg or placebo through Week 16. Placebo patients transitioned to ICO after Week 16. Overall ICONIC-LEAD co-primary endpoints, IGA 0/1 response (IGA score=cleared [0] or minimal [1] and ≥2-grade improvement) and PASI 90 response at Week 16, were assessed in adolescents, as were the efficacy and safety through Week 24.
Results: Adolescents comprised 9.6% (66/684) of the ICONIC-LEAD population. The mean age was 15 years and the mean psoriasis disease duration was 5.2 years (Table). Higher proportions of adolescents receiving ICO vs placebo achieved clear/almost clear skin at Week 16 (IGA 0/1: 84.1% vs 27.3%; PASI 90: 70.5% vs 13.6%; nominal p< 0.001). At Week 24, rates of clear/almost clear skin with ICO were 86.4% (IGA 0/1) and 88.6% (PASI 90; Figure). Response patterns were consistent for IGA 0 and PASI 100. At Week 16, 50% of ICO and 73% of placebo adolescents had ≥1 adverse event; no safety signals emerged through Week 24.
Conclusion: In adolescents with moderate-to-severe psoriasis, higher proportions of ICO vs placebo participants achieved clear/almost clear skin at Week 16, with unprecedented response rates at Week 24 and a favorable adverse event profile.
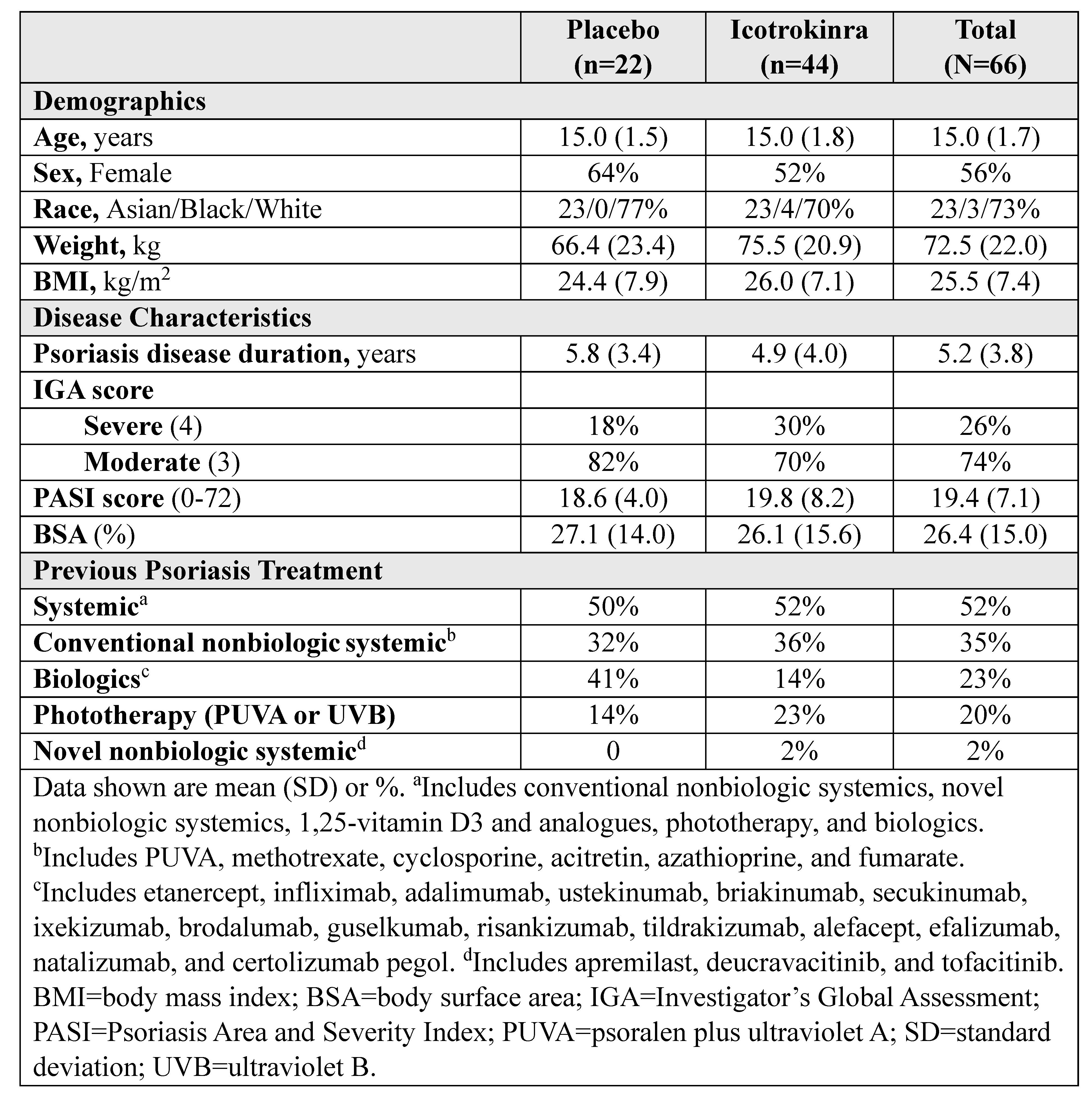 Table. Baseline Demographics, Disease Characteristics, and Previous Treatment Among Adolescents With Moderate-to-Severe Psoriasis
Table. Baseline Demographics, Disease Characteristics, and Previous Treatment Among Adolescents With Moderate-to-Severe Psoriasis
.jpg) Figure. IGA 0/1 and PASI 90 Response at W16 and W24
Figure. IGA 0/1 and PASI 90 Response at W16 and W24
To cite this abstract in AMA style:
Eichenfield L, Galimberti R, Hebert A, Wang W, Soung J, Magnolo N, Browning J, Moore A, Lebwohl M, Wilsmann-Theis D, Merola J, Kokolakis G, Mortazawi D, Grewal P, Miller M, Cafone J, Li S, Jiang G, Nunes F, DeKlotz C, Paller A. Efficacy and Safety of Icotrokinra, a Novel Targeted Oral Peptide (IL-23R-Inhibitor), in Adolescents With Moderate-to-Severe Plaque Psoriasis: Subgroup Analyses From a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study (ICONIC-LEAD) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-icotrokinra-a-novel-targeted-oral-peptide-il-23r-inhibitor-in-adolescents-with-moderate-to-severe-plaque-psoriasis-subgroup-analyses-from-a-phase-3-randomized-double-blin/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-icotrokinra-a-novel-targeted-oral-peptide-il-23r-inhibitor-in-adolescents-with-moderate-to-severe-plaque-psoriasis-subgroup-analyses-from-a-phase-3-randomized-double-blin/
