Session Information
Date: Monday, October 27, 2025
Title: Plenary II (0849–0854)
Session Type: Plenary Session
Session Time: 9:00AM-9:15AM
Background/Purpose: Fetal atrioventricular block (fAVB) is presumed dependent on the transplacental passage of SSA/Ro52/60kD autoantibodies that bind to apoptotic fetal cardiomyocytes, triggering macrophage activation and cardiac fibrosis. While anti-Ro is necessary for fAVB, titers alone are insufficient for prediction which challenges preconception counseling, surveillance, and trial design. Thus, identification of additional permissive factors for injury would yield greater understanding of pathogenesis and more precise biomarkers of significant clinical utility. The biobanks generated from the STOP BLOQ and PATCH trials represent a rare opportunity to evaluate sera collected during pregnancy but prior to fAVB occurrence. Accordingly, this study leveraged these samples and Olink proteomics to identify additional predictive serum proteins for fAVB in high titer anti-Ro pregnant subjects.
Methods: Sera from 12 fAVB affected pregnancies were matched to 13 high titer anti-Ro exposed healthy pregnancies. To eliminate factors conferring a change in serum proteins and/or fAVB risk, samples were matched on race/ethnicity, maternal rheumatologic diagnosis, anti-Ro52 and 60 titers (ELISA in our research lab), and hydroxychloroquine dose. All samples were collected prospectively between weeks 10-19 during enrollment in STOP BLOQ or PATCH and, for affected pregnancies, before the development of fAVB. Samples from five anti-Ro negative healthy pregnant women were also tested. Serum proteins ( >5400) were assessed by the Olink Explore HT assay. Principal component analysis (PCA) was performed and a t-test was employed to compare fAVB to anti-Ro exposed healthy pregnancies. Candidate proteins had a p< 0.01 and log2FC |1|.
Results: The 12 fAVB pregnancies and 13 anti-Ro+ non-fAVB pregnancies were well matched on clinical variables (Table 1). As a validation, high levels of HCG-beta were detected (Fig 1A). The PCA effectively distinguished anti-Ro+ from anti-Ro- pregnancies and asymptomatic or undifferentiated autoimmune syndrome (Asym/UAS) from SjD/SLE, but did not separate fAVB from non-fAVB cases (Fig 1B & 1C). While 78 proteins separated Asym/UAS from SjD/SLE, there were 15 other differentially abundant proteins between fAVB vs anti-Ro+ non-fAVB pregnancies (Fig 1D & 2). That these 15 proteins did not differ between UAS and SjD/SLE pregnancies supports prior observations that maternal diagnosis does not influence fAVB risk (Fig 1E & 1F). Two macrophage related proteins, IL6 (log2FC = 4.1) and CCL3 (log2FC = 3.9), showed the greatest difference between fAVB and non-fAVB subjects (Fig 2), and were highly correlated (r=0.84, p< 0.0001). Neither were correlated with either anti-Ro52 or 60 levels.
Conclusion: This high-throughput evaluation of prospective samples collected early in pregnancy provides insights into novel proteins that predict the development of fAVB in anti-Ro exposed pregnancies. Given the low molecular weight of these novel pro-inflammatory candidates, passive transport across the placenta supports an additional maternal contribution (not related to maternal disease status which was separated by other proteins) to the pathogenesis by amplifying anti Ro-mediated fetal cardiac injury.
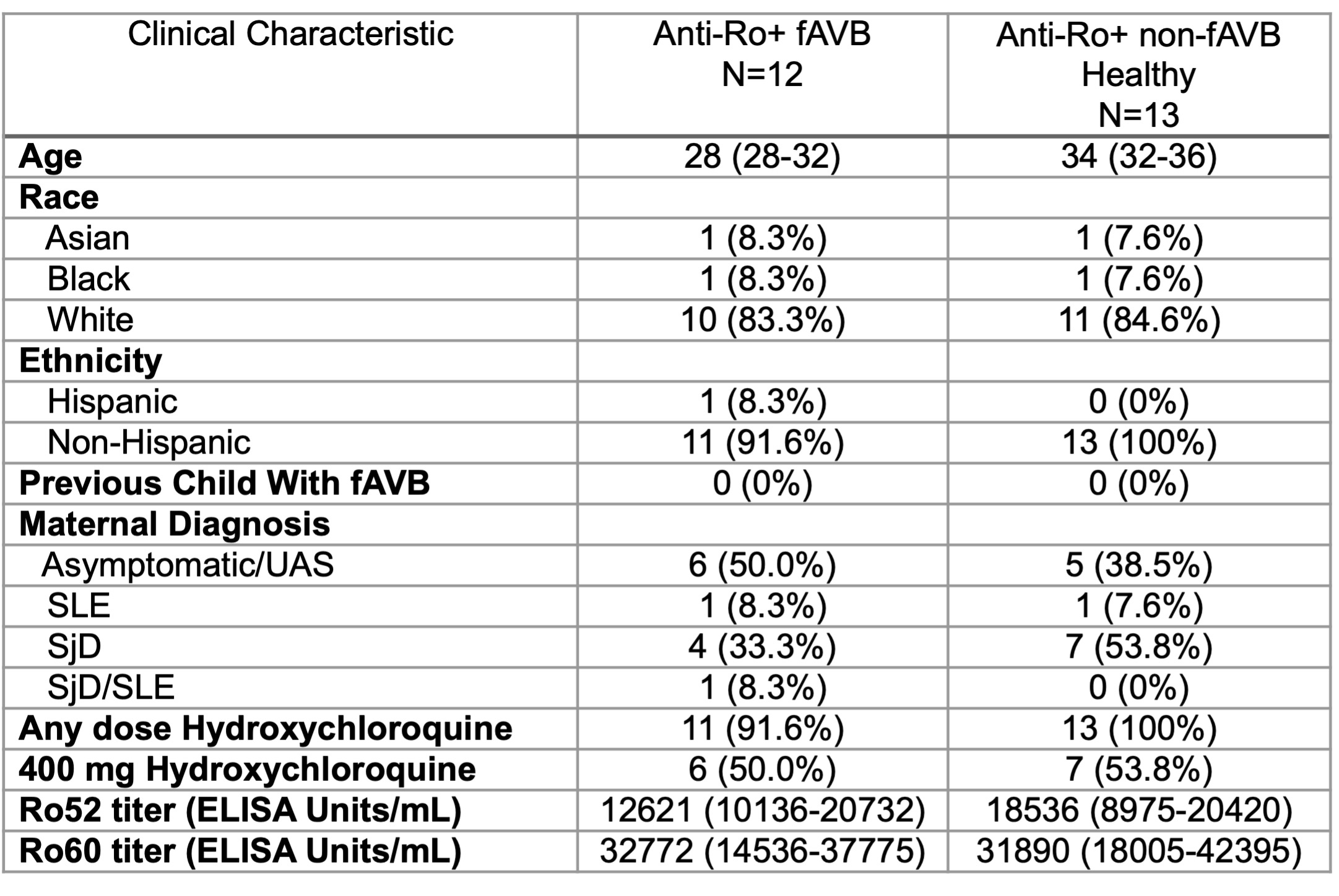 Table 1: Clinical characteristics of fAVB and Anti-Ro+ non fAVB pregnancies. Data are represented as median (IQR) or N (%). fAVB: fetal atrioventricular block, SLE: Systemic Lupus Erythematosus, SjD: Sjögren’s Disease, UAS: Undifferentiated Autoimmune Syndrome
Table 1: Clinical characteristics of fAVB and Anti-Ro+ non fAVB pregnancies. Data are represented as median (IQR) or N (%). fAVB: fetal atrioventricular block, SLE: Systemic Lupus Erythematosus, SjD: Sjögren’s Disease, UAS: Undifferentiated Autoimmune Syndrome
.jpg) Figure 1: A) Boxplots showing the levels of HCG-beta detected among the fAVB, anti-Ro+ healthy, and anti-Ro- healthy pregnancies. Note that one healthy anti-Ro negative control is not represented due to a QC failure for the HCG-beta assay. The horizontal red dashed line represents the technical lower limit of detection for HCG-beta provided by Olink. B) PCA visualization of the fAVB, anti-Ro+ healthy, and anti-Ro- healthy pregnancies. C) PCA visualization of the Asym/UAS, SjD/SLE, and anti-Ro- healthy pregnancies. D) Volcano plot showing the 15 differentially abundant proteins between fAVB and anti-Ro+ healthy subjects. E) Volcano plot showing the 78 differentially abundant proteins between SjD/SLE and Asym/UAS subjects. F) Venn diagram showing that none of the 78 differentially abundant proteins between SjD/SLE and Asym/UAS subjects overlap with the 15 differentially abundant proteins between fAVB and anti-Ro+ healthy subjects.
Figure 1: A) Boxplots showing the levels of HCG-beta detected among the fAVB, anti-Ro+ healthy, and anti-Ro- healthy pregnancies. Note that one healthy anti-Ro negative control is not represented due to a QC failure for the HCG-beta assay. The horizontal red dashed line represents the technical lower limit of detection for HCG-beta provided by Olink. B) PCA visualization of the fAVB, anti-Ro+ healthy, and anti-Ro- healthy pregnancies. C) PCA visualization of the Asym/UAS, SjD/SLE, and anti-Ro- healthy pregnancies. D) Volcano plot showing the 15 differentially abundant proteins between fAVB and anti-Ro+ healthy subjects. E) Volcano plot showing the 78 differentially abundant proteins between SjD/SLE and Asym/UAS subjects. F) Venn diagram showing that none of the 78 differentially abundant proteins between SjD/SLE and Asym/UAS subjects overlap with the 15 differentially abundant proteins between fAVB and anti-Ro+ healthy subjects.
.jpg) Figure 2: Boxplots showing the levels of the 15 significantly different proteins with p < 0.01 and log2FC < -1 or > 1 between fAVB and anti-Ro+ healthy subjects.
Figure 2: Boxplots showing the levels of the 15 significantly different proteins with p < 0.01 and log2FC < -1 or > 1 between fAVB and anti-Ro+ healthy subjects.
To cite this abstract in AMA style:
Carlucci P, Masson M, Phoon C, Roman A, Izmirly P, Saxena A, Belmont M, Penfield C, Lee Y, Nusbaum J, Rubenstein A, Sachan N, Guthridge J, James J, Sinkovskaya E, Abuhamad A, Satou G, Hogan W, Moon-Grady A, Howley L, Donofrio M, Levasseur S, Geiger M, Owens S, Cumbermack K, Matta J, Joffe G, Lindblade C, Haxel C, Kohari K, Copel J, Strainic J, Doan T, Holloman C, Killen S, Tacy T, Kaplinski M, Fraser N, Ruggles K, Cuneo B, Buyon J, Clancy R. Olink Proteomics Identifies Macrophage Pro-inflammatory Proteins in Maternal Sera Predictive of Fetal Atrioventricular Block Independent of Maternal Health Status [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/olink-proteomics-identifies-macrophage-pro-inflammatory-proteins-in-maternal-sera-predictive-of-fetal-atrioventricular-block-independent-of-maternal-health-status/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/olink-proteomics-identifies-macrophage-pro-inflammatory-proteins-in-maternal-sera-predictive-of-fetal-atrioventricular-block-independent-of-maternal-health-status/
