Session Information
Session Type: Abstract Session
Session Time: 4:15PM-4:30PM
Background/Purpose: Our ability to tailor treatments to individual patients with JIA remains limited. To identify candidate biomarkers that may be associated with treatment response, we characterized circulating lymphocytes in patients treated with abatacept in the Improving Outcomes in Limited JIA (LIMIT-JIA) study.
Methods: LIMIT-JIA is a multicenter trial conducted within the Childhood Arthritis and Rheumatology Research Alliance (CARRA) comparing abatacept treatment for 24 weeks to usual care in patients with limited arthritis (< 5 joints at disease onset). Study subjects were diagnosed within 6 months, had no history of uveitis, and were DMARD naïve. Blood samples were collected from 12 different CARRA sites at baseline (before treatment) and at 6 months and shipped to the CARRA Biobank for cryopreservation. Peripheral blood mononuclear cells (PBMCs) were then thawed, stained with antibodies from 2 panels to characterize T/B/NK cells (48 markers/panel), barcoded for mass cytometry, and acquired on a CyTOF XT instrument. Samples were processed in 5 batches with each batch containing samples from multiple study groups/time points. After compensation and debarcoding, CyCombine corrected and integrated the results from the batches. OMIQ software was used for unsupervised clustering with FlowSOM and results were visualized with a uniform manifold approximation and projections (UMAP) and heatmaps. EdgeR in OMIQ was used for statistical analysis.
Results: Patients in the abatacept (n=36) and usual care (n=14) arms provided paired samples at baseline and 6 months. T, B, NK, and myeloid cells were identified with data from each panel (Fig 1A, 2A). After gating on CD3+ or CD56+ cells, 23 clusters of T/NK cells were identified (Fig 1B-C). Three clusters were significantly more abundant in patients at baseline compared to those treated with abatacept at 6 months. These clusters contained cells with features of regulatory T (Treg) (cluster 5), T follicular helper (Tfh) (cluster 6), and double negative (DN) T cells (cluster 10) (Fig 1C-D). There was no difference in cluster distribution in patients at baseline vs. those receiving usual care at 6 months (Fig 1D). When comparing patients at 6 months, T cells were more frequent in the Tfh cell cluster in the usual care vs. abatacept groups (Fig 1D). Clustering on CD20+CD19+cells revealed 26 different B cell populations (Fig 2B). Cluster 10 contained CD21lo B cells with features of age-/autoimmune-associated B cells (ABCs) and was overrepresented in patients at baseline compared to those treated with abatacept at 6 months. There was no difference in B cell cluster abundance in patients at baseline vs. usual care at 6 months. Cluster 11, containing unswitched memory B cells, was enriched in usual care vs. abatacept at 6 months.
Conclusion: Abatacept treatment in JIA patients with limited arthritis modulated the frequency of circulating Treg, Tfh, DN T, and CD21loB cells. These results suggest that interactions between B cell helper T cells and CD21loB cells are important in the pathogenesis of this form of arthritis and may be regulated by T cell costimulatory inhibition. Further studies are needed to determine if these lymphocyte populations can predict and track treatment response in JIA patients.
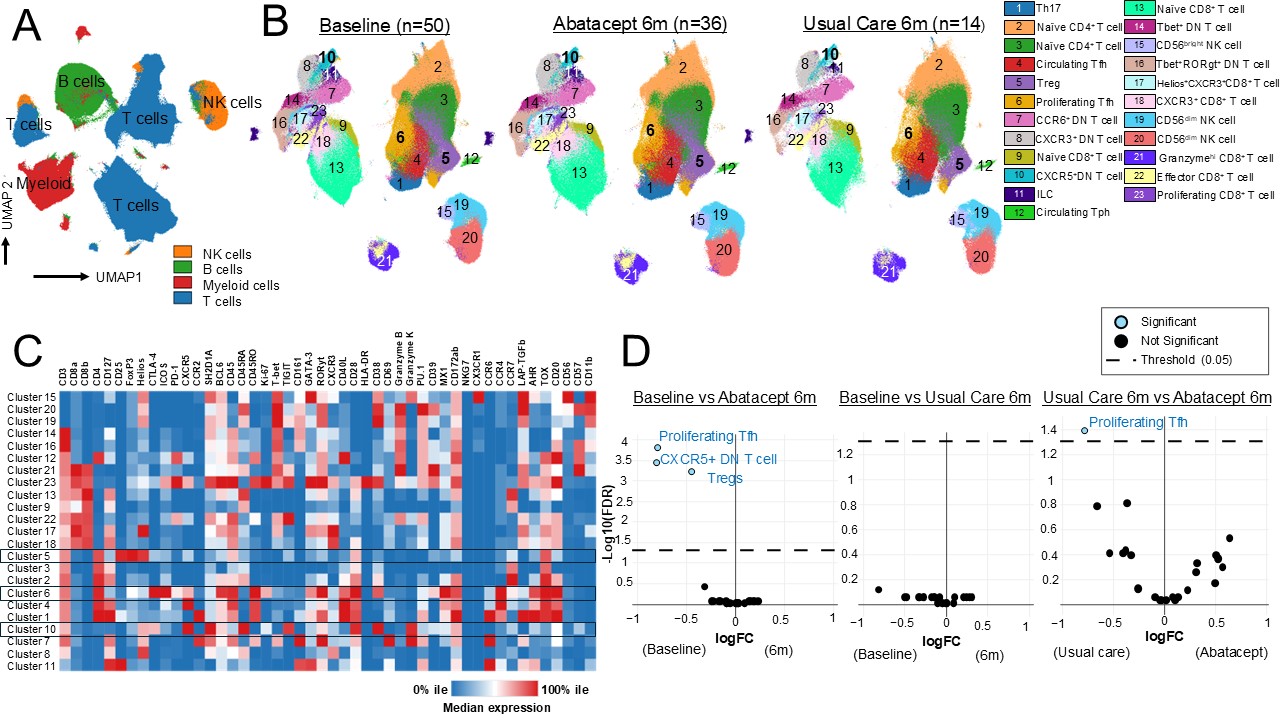 Figure 1: Abatacept treatment modulates circulating T cell subsets in patients with limited JIA. A) UMAPs generated from mass cytometry data of the 48-marker panel used to immunophenotype T and NK cells. Samples were from JIA patients at baseline (n=50) and those treated with abatacept (n=36) or usual care (n=14) at 6 months. B) UMAPs generated after filtering for T and NK cells and unsupervised clustering. C) Heatmap showing the median expression of a given marker in each cluster. D) Volcano plots showing differential abundance of the clusters in the given study groups.
Figure 1: Abatacept treatment modulates circulating T cell subsets in patients with limited JIA. A) UMAPs generated from mass cytometry data of the 48-marker panel used to immunophenotype T and NK cells. Samples were from JIA patients at baseline (n=50) and those treated with abatacept (n=36) or usual care (n=14) at 6 months. B) UMAPs generated after filtering for T and NK cells and unsupervised clustering. C) Heatmap showing the median expression of a given marker in each cluster. D) Volcano plots showing differential abundance of the clusters in the given study groups.
JIA, juvenile idiopathic arthritis; UMAPs, uniform manifold approximation and projections; mo, months; Treg, regulatory T; Tfh, T follicular helper; Tph, T peripheral helper; DN T, double negative T
.jpg) Figure 2: Abatacept treatment decreases circulating CD21lo B cells in patients with limited JIA. A) UMAPs generated from mass cytometry data of the 48-marker panel used to immunophenotype B cells. Samples were from JIA patients at baseline (n=50) and those treated with abatacept (n=36) or usual care (n=14) at 6 months. B) UMAPs generated after filtering for B cells and unsupervised clustering. C) Heatmap showing the median expression of a given marker in each cluster. D) Volcano plots showing differential abundance of the clusters in the given study group.
Figure 2: Abatacept treatment decreases circulating CD21lo B cells in patients with limited JIA. A) UMAPs generated from mass cytometry data of the 48-marker panel used to immunophenotype B cells. Samples were from JIA patients at baseline (n=50) and those treated with abatacept (n=36) or usual care (n=14) at 6 months. B) UMAPs generated after filtering for B cells and unsupervised clustering. C) Heatmap showing the median expression of a given marker in each cluster. D) Volcano plots showing differential abundance of the clusters in the given study group.
JIA, juvenile idiopathic arthritis; UMAPs, uniform manifold approximation and projections; mo, months; ABC, age-/autoimmune-associated B cells; DN, double negative; Bmem, memory B cell
To cite this abstract in AMA style:
AitDowd L, Murzin E, Pommier A, Lam K, Harris C, Kohlheim M, Schulert G, Sudman M, Wu E, Schanberg L, Nigrovic P, Lederer J, Henderson L. Characterizing Immune Responses in Abatacept-treated Patients with Limited Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/characterizing-immune-responses-in-abatacept-treated-patients-with-limited-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterizing-immune-responses-in-abatacept-treated-patients-with-limited-juvenile-idiopathic-arthritis/
