Session Information
Session Type: Abstract Session
Session Time: 3:15PM-3:30PM
Background/Purpose: Cardiovascular disease (CVD) is a leading cause of morbidity and mortality in juvenile-onset systemic lupus erythematosus (JSLE). There is an urgent need to identify biomarkers that can predict atherosclerosis progression and therapeutic responses, enabling personalised CVD-risk management in JSLE. This study aimed to investigate whether novel autoantibody signatures can predict atherosclerosis progression and atorvastatin response in children and young people with JSLE.
Methods: We conducted a biomarker discovery study using baseline serum samples from a sub-cohort of the APPLE trial (Atherosclerosis Prevention in Paediatric Lupus Erythematosus), a multi-centre, randomised, double-blind, placebo-controlled clinical trial of atorvastatin versus placebo (1:1) to prevent atherosclerosis progression in JSLE, conducted across 21 sites in North America. Unsupervised cluster analysis based on carotid intima media thickness (CIMT) progression over 36 months was used to identify groups with high and low atherosclerosis progression within each treatment arm. Baseline differential autoantibody expression was assessed using Empirical Bayes moderated t-tests. Predictive performance was evaluated using logistic regression and ROC analysis.
Results: Ninety-four JSLE patients (mean [SD] age 15.3 [2.4] years; 78% female), with matched baseline serum samples and complete longitudinal CIMT measurements over 36 months, were included (45 randomized to the placebo and 49 to the atorvastatin arm) (Table 1). A total of 579 autoantibodies were identified as true signals in the 94 baseline serum samples analysed. In the placebo arm, six autoantibodies (STK24, RAD23B, HDAC4, STAT4, SEPTIN9 and NFIA) were significantly associated with high versus low CIMT progression over 36 months in the placebo arm, achieving a combined area under the curve (AUC) of 87% (Figure 1A-C). In the atorvastatin arm, a distinct autoantibody profile was identified, which predicted response to statin treatment. Eight autoantibodies (ABI1, ATP5B, CSNK2A2, NRIP3, PRKAR1A, PDK4, BATF and NUDT2) were significantly associated with CIMT progression despite atorvastatin therapy, achieving an exceptional combined AUC of 96% (Figure 1D-F). Enriched pathway analysis of the autoantibodies that differentiated responders vs. non-responders to atorvastatin revealed lipid-independent mechanisms, such as accumulation of vascular smooth muscle cells within neointima (CSNK2A2) or vascular calcification and regulation of cell proliferation/apoptosis (PDK4).Based on the predictive models generated from these distinct autoantibody profiles in both the placebo and atorvastatin arms, a two-step stratification strategy was proposed to enable precision risk assessment and guide treatment decisions for CVD-risk management in JSLE (Figure 2).
Conclusion: In this biomarker discovery cohort study, novel autoantibody signatures were identified as the first JSLE-specific serum markers for atherosclerosis progression and prediction of statin response (currently under patent protection). These findings support the potential application of autoantibody profiling for precision medicine approaches for CVD-risk management in JSLE.
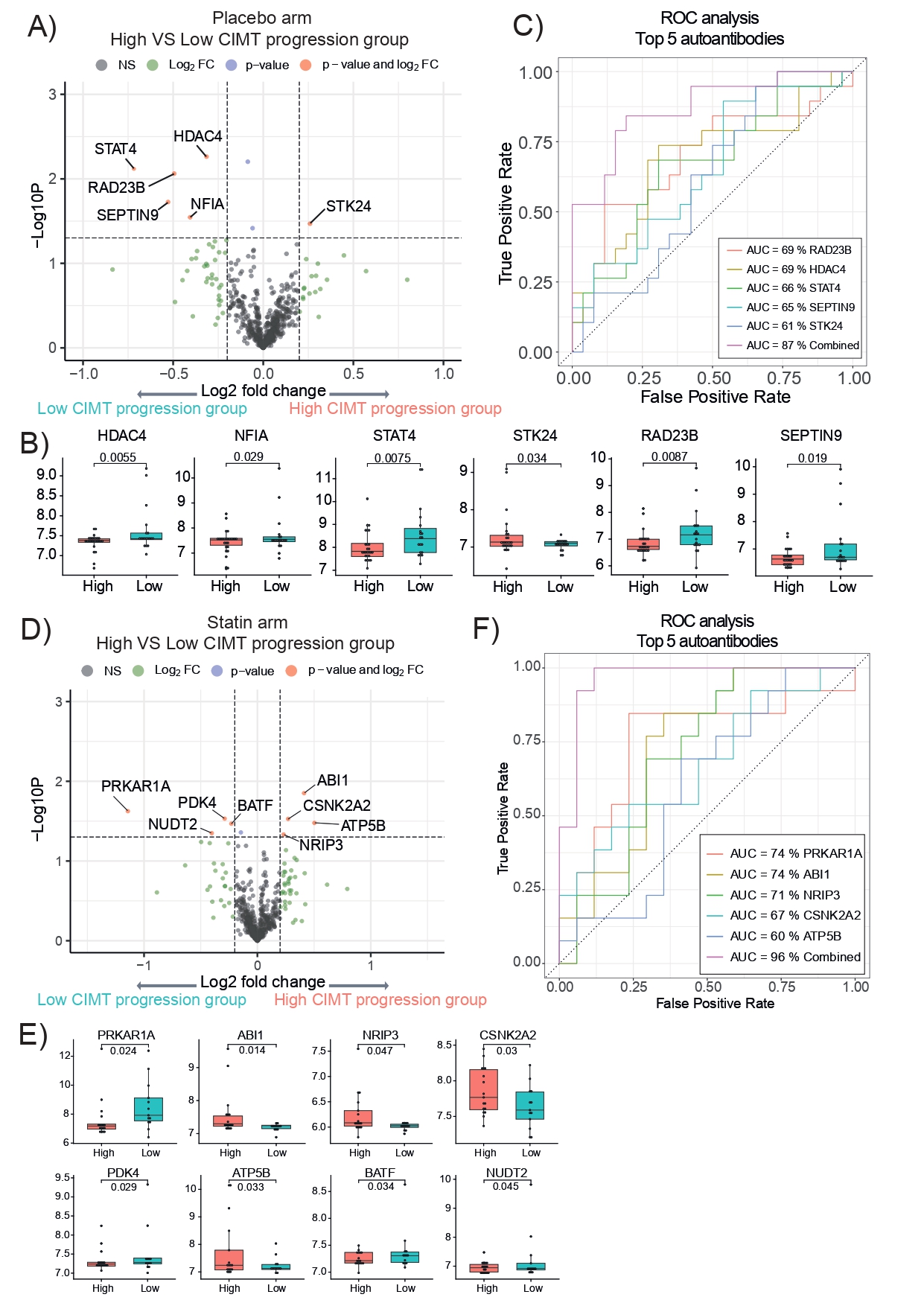 Table 1 Demographic/clinical table of the APPLE trial sub-cohort (Nf94) in the autoantibody profiling study. Abbreviations: BMI – Body mass index; SLEDAI – Systemic Lupus Erythematosus Disease Activity Index; SLICC/ACR-DI – Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; dsDNA antibody – Anti-double-stranded DNA antibody; C3, C4 – complement fractions C3, C4; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
Table 1 Demographic/clinical table of the APPLE trial sub-cohort (Nf94) in the autoantibody profiling study. Abbreviations: BMI – Body mass index; SLEDAI – Systemic Lupus Erythematosus Disease Activity Index; SLICC/ACR-DI – Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; dsDNA antibody – Anti-double-stranded DNA antibody; C3, C4 – complement fractions C3, C4; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
.jpg) Figure 1: Juvenile-onset systemic lupus erythematosus (JSLE) patient stratification by ΔCIMT at baseline versus 36 months and autoantibody profiling in the placebo and statin arms of the APPLE trial. A, D. Volcano plot displaying fold change of all autoantibodies and Log10 p values comparing high and low CIMT progression groups – placebo and statin arm (p < 0.05; log2(fold change)>0.2). B, E. Box and whisker plots showing the significant autoantibody levels of the high vs. low CIMT progression groups – placebo and statin arms. Empirical Bayes moderated t-test. C, F. Individual and combined ROC analysis for discriminating high vs. low CIMT progression groups in both in the placebo and statin arms of the APPLE trial using the top performing autoantibodies (individual AUC > 60%). Legend: Autoantibodies defining the atherosclerosis progression signature: RAD23B – RAD23 homolog B, HDAC4 – Histone Deacetylase 4; STAT4 – Signal Transducer and Activator of Transcription 4; STK24 – Serine/Threonine kinase 24; Autoantibodies defining the statin response signature: PRKAR1A – cAMP-dependent protein kinase type I-alpha regulatory subunit; ABI1- Abelson interactor 1; NRIP3 – Nuclear receptor interacting protein 3; CSNK2A2 – Casein kinase II subunit alpha; PDK4 – Pyruvate Dehydrogenase kinase isozyme 4; ATP5B – ATP synthase subunit beta; BATF -Basic leucine zipper transcription factor ATF-like; NUDT2 – Nudix Hydrolase 2.
Figure 1: Juvenile-onset systemic lupus erythematosus (JSLE) patient stratification by ΔCIMT at baseline versus 36 months and autoantibody profiling in the placebo and statin arms of the APPLE trial. A, D. Volcano plot displaying fold change of all autoantibodies and Log10 p values comparing high and low CIMT progression groups – placebo and statin arm (p < 0.05; log2(fold change)>0.2). B, E. Box and whisker plots showing the significant autoantibody levels of the high vs. low CIMT progression groups – placebo and statin arms. Empirical Bayes moderated t-test. C, F. Individual and combined ROC analysis for discriminating high vs. low CIMT progression groups in both in the placebo and statin arms of the APPLE trial using the top performing autoantibodies (individual AUC > 60%). Legend: Autoantibodies defining the atherosclerosis progression signature: RAD23B – RAD23 homolog B, HDAC4 – Histone Deacetylase 4; STAT4 – Signal Transducer and Activator of Transcription 4; STK24 – Serine/Threonine kinase 24; Autoantibodies defining the statin response signature: PRKAR1A – cAMP-dependent protein kinase type I-alpha regulatory subunit; ABI1- Abelson interactor 1; NRIP3 – Nuclear receptor interacting protein 3; CSNK2A2 – Casein kinase II subunit alpha; PDK4 – Pyruvate Dehydrogenase kinase isozyme 4; ATP5B – ATP synthase subunit beta; BATF -Basic leucine zipper transcription factor ATF-like; NUDT2 – Nudix Hydrolase 2.
CIMT- carotid intima media thickness
.jpg) Figure 2: Algorithm for potential use of the novel autoantibody signatures in clinical practice for stratification of JSLE patient population based on CVD-risk and prediction of response to statin treatment.
Figure 2: Algorithm for potential use of the novel autoantibody signatures in clinical practice for stratification of JSLE patient population based on CVD-risk and prediction of response to statin treatment.
To cite this abstract in AMA style:
Peng J, Donnes P, McDonnell T, Schanberg L, Ardoin S, Lewandowski L, Robinson G, C Jury E, Ciurtin C. Novel Autoantibodies Predictive of Atherosclerosis Progression and Statin Response in Juvenile-Onset Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/novel-autoantibodies-predictive-of-atherosclerosis-progression-and-statin-response-in-juvenile-onset-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-autoantibodies-predictive-of-atherosclerosis-progression-and-statin-response-in-juvenile-onset-systemic-lupus-erythematosus/
