Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes (0789–0794)
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: Patients with idiopathic inflammatory myopathies (IIM) experience significant impairment in their health-related quality of life (QoL); however, there are currently no validated measures to assess QoL in these patients. This study aims to examine the measurement properties of short form (SF)-36 in QoL assessment of adults with IIM.
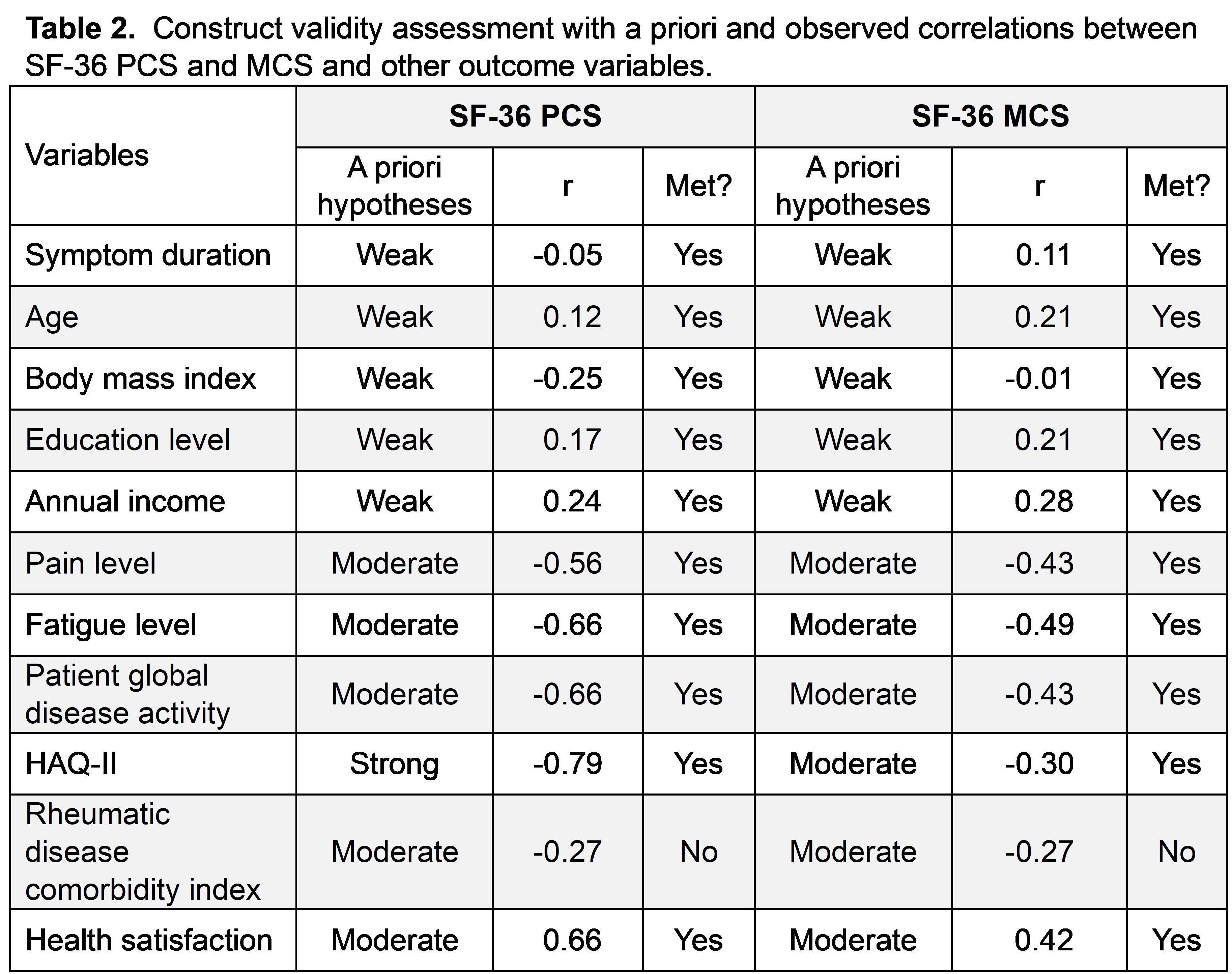
Methods: FORWARD is a U.S.-based databank collecting biannual patient-reported data on rheumatic diseases, including sociodemographics, health-care utilization and forms related to patients’ symptoms and experiences including patient global disease activity (visual analog scale [VAS]), pain (VAS), fatigue (VAS), physical functioning (Health Assessment Questionnaire [HAQ-II]), health satisfaction and quality of life (SF-36). SF-36 produces physical (PCS) and mental (MCS) component scores, ranging 0-100 with higher scores indicating a better QoL. Floor and ceiling effects were calculated based on the proportion of patients who have the lowest and the highest possible SF-36 score, respectively. Discriminant validity was assessed by comparing the median subgroups of patients using t test. Construct validity was assessed by Pearson correlation between the SF-36 and other relevant outcome variables based on a priori hypotheses. Adequate validity was defined as ≥70% agreement between the results and a priori hypotheses. Responsiveness was assessed using linear mixed models to examine the relationship between change in SF-36 and other outcome variables over time.
Results: A total of 168 patients with IIM were included (77.3% female, 78.5% White), with an average (± standard deviation [SD]) age of 54.3 (±13.8). No floor or ceiling effect was observed for SF-36 PCS and MCS. SF-36 PCS was significantly different between median groups with low vs high physical functioning, disease activity, fatigue and pain, while MCS was significantly different between patients with and without depression and anxiety, and low vs high fatigue and pain levels (Table 1). Over 90% of a priori hypotheses for construct validity were met (Table 2). SF-36 PCS and MCS had moderate to strong correlations with pain, fatigue, physical function, disease activity, and health satisfaction. Longitudinal changes in SF-36 were significantly associated with changes in in pain, fatigue, patient global disease activity, and physical functioning (Table 3).
Conclusion: SF-36 had no significant floor or ceiling effect and demonstrated adequate validity and responsiveness in patients with IIM. These results support using SF-36 for health-related QoL assessment in patients with IIM.
To cite this abstract in AMA style:
Razok A, Taylor J, Ritz E, Wipfler K, Michaud K, Saygin D. Short Form 36 (SF-36) Health Survey Questionnaire in Health-Related Quality of Life Assessment in Patients with Inflammatory Myopathies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/short-form-36-sf-36-health-survey-questionnaire-in-health-related-quality-of-life-assessment-in-patients-with-inflammatory-myopathies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-form-36-sf-36-health-survey-questionnaire-in-health-related-quality-of-life-assessment-in-patients-with-inflammatory-myopathies/

.jpg)
.jpg)