Session Information
Date: Sunday, October 26, 2025
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: VEXAS syndrome is a recently described severe disease characterized by a complex overlap of inflammatory and hematologic features. Due to the severity and refractory nature of inflammation in VEXAS patients, establishing effective treatment strategies is challenging, particularly in the absence of validated tools to assess disease activity. Standardized disease activity indices (DAIs) are crucial for patient care and advancing clinical research in inflammatory disorders. To date, the only proposed – though not validated – DAI for VEXAS is the VEXASCAF, which evaluates disease activity based on the presence or absence of 11 equally weighted manifestations (Kirino Y, et al. Rheumatology (Oxford). 2024 Sep 28). While the VEXASCAF represents an important first step, it has limitations: it may not capture less commonly involved organ systems or be sufficiently sensitive to changes in disease severity. A more comprehensive and sensitive DAI could aid in clinical research and improve patient care.
Methods: Manifestations of VEXAS syndrome were previously identified by systematic literature review and expert feedback. The VEXAS-DAI was developed based on an expert advisory committee using modified Delphi methodology until consensus was reached (defined as ≥75% concurrence). All items were graded based on adapted Common Terminology Criteria for Adverse Events (CTCAE) criteria. Scoring was developed based on Physician Global Assessment of inflammation on 158 test cases. The final instrument was developed based on input from 24 VEXAS expert physicians worldwide.
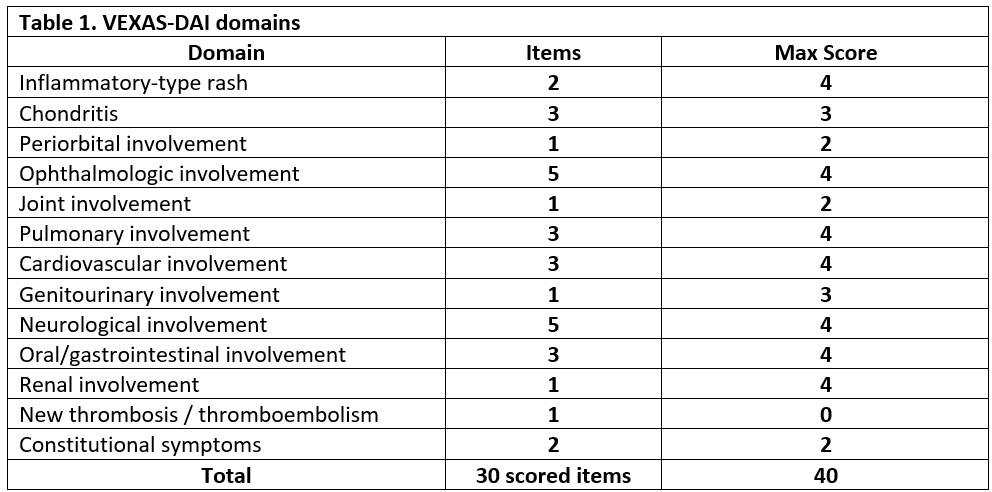
Results: Consensus on the novel VEXAS-DAI was achieved after 4 rounds of interactive revisions. Scoring is based on severity of symptoms at the time of examination. The VEXAS-DAI includes 12 scored domains, each with a specified number of items (Table 1): inflammatory-type rash (2), chondritis (3), ophthalmologic involvement (5), periorbital involvement (1), joint (1), pulmonary (3), cardiovascular (3), genitourinary (1), neurologic (5), oral and gastrointestinal (3), renal (1), and constitutional symptoms (present or absent). Additionally, a thrombosis and thromboembolism domain is included but remains unscored. Scores range from 0 to 40.
Conclusion: The VEXAS-DAI is designed to measure active inflammation in patients with VEXAS syndrome. Work is ongoing to validate this instrument in the context of a clinical trial.This abstract has been accepted for publication by the European Alliance of Associations for Rheumatology (EULAR). The definitive copyedited version is available online at: www.ard.eular.org.
To cite this abstract in AMA style:
Byram K, Mann H, Hammond D, Savic S, Kirino Y, Gurnari C, Heiblig M, Comont T, Mekinian A, Patnaik M, Weeks L, Ho G, Chowdhury O, Al-Hakim A, Goldberg S, ferrada M, georgin-Lavialle S, Grayson P, Groarke E, Patel B, Sullivan M, Buckley S, harder B, Goble S, Koster M, Beck D. Development of a Disease Activity Index for the Assessment of VEXAS Syndrome (VEXAS-DAI) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-of-a-disease-activity-index-for-the-assessment-of-vexas-syndrome-vexas-dai/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-disease-activity-index-for-the-assessment-of-vexas-syndrome-vexas-dai/