Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic autoimmune vasculitis. Avacopan, a novel oral C5a receptor inhibitor, has emerged as an adjunct therapy for AAV. However, a notable side effect of avacopan is drug-induced liver injury (DILI). This study aims to assess the risk of DILI associated with avacopan in patients with AAV.
Methods: We conducted a retrospective cohort study using the TriNetX Research Network, a de-identified, federated electronic health record database (accessed April 16, 2025). Adult patients (≥18 years) with AAV (ICD-10: M31.3, M31.7, I77.82) and DILI (K71.0, K71.1, K71.2) were included. A 1:1 propensity score matching was performed using demographics, comorbidities, and CYP3A4 inhibitor medication exposures to balance baseline characteristics of patients on standard of care treatment with or without avacopan. Continuous and categorical variables were compared using t-tests and chi-squared tests (α = 0.05).
Results: A total of 29,163 patients with ANCA-associated vasculitis (AAV) were identified, including 522 treated with avacopan and 28,641 who were not. Before matching, significant differences were observed between groups in several demographic and clinical characteristics. The avacopan group had higher proportions of Hispanic patients (11.5% vs. 8.5%; p = 0.017) (Table 1). Comorbid conditions such as diabetes mellitus, nonalcoholic steatohepatitis (NASH), metabolic syndrome, chronic viral hepatitis, and essential hypertension were all more common among those receiving avacopan (all p < 0.05). Additionally, avacopan group had significantly higher use CYP3A4 inhibitors including itraconazole, ketoconazole, fluconazole, posaconazole, voriconazole, nirmatrelvir, ritonavir, amiodarone, diltiazem, cimetidine, and cyclosporine (all p < 0.05) (Table 2). Following 1:1 propensity score matching, 516 patients remained in each group. No statistically significant differences were observed in age, sex, race, ethnicity, comorbidities, or medication use. The incidence of DILI was nearly identical between the two groups (2.0% vs. 1.9%), with a risk difference of 0.0% (95% CI: –0.017 to 0.017; p = 0.975). There was no significant difference in DILI-free survival between groups (log-rank p = 0.873), and the hazard ratio for time to DILI was 1.25 (95% CI: 0.078–20.07; p = 0.171).
Conclusion: In this matched cohort analysis, the incidence of DILI was low and comparable between patients treated with avacopan and those who were not. While no significant difference was observed, the limited number of events precludes definitive conclusions regarding comparative risk.
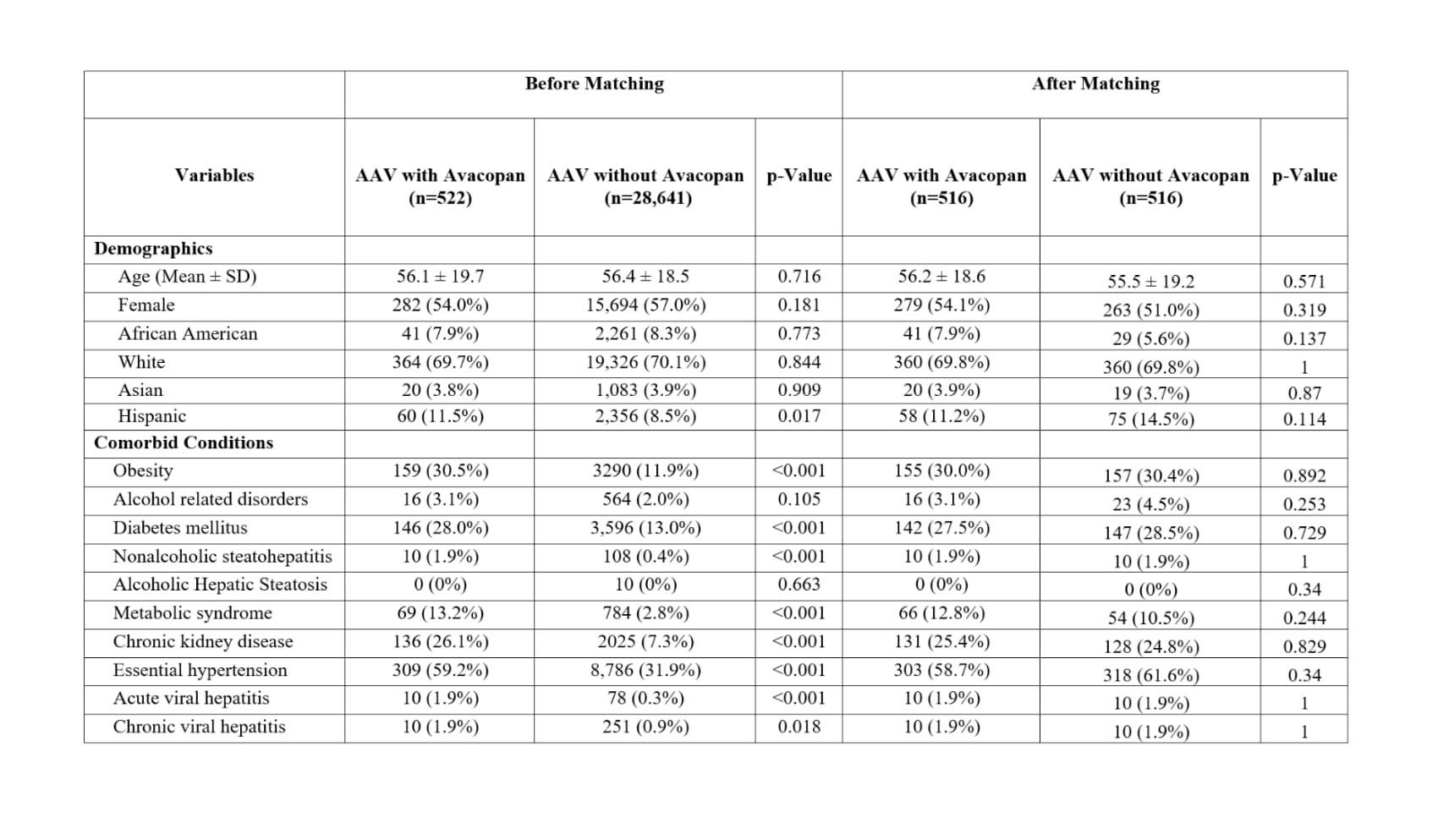 Table 1: Baseline demographics and comorbidities in patients with AAV treated with or without avacopan before and after propensity score matching.
Table 1: Baseline demographics and comorbidities in patients with AAV treated with or without avacopan before and after propensity score matching.
.jpg) Table 2: Use of CYP3A4 inhibitors in patients with AAV treated with or without avacopan, before and after propensity score matching.
Table 2: Use of CYP3A4 inhibitors in patients with AAV treated with or without avacopan, before and after propensity score matching.
To cite this abstract in AMA style:
Heydari-Kamjani M, Harper E, Chaudhary H. Risk of Drug Induced Liver Injury with Use of Avacopan in ANCA Vasculitis – Results from Real-World Data [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-of-drug-induced-liver-injury-with-use-of-avacopan-in-anca-vasculitis-results-from-real-world-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-drug-induced-liver-injury-with-use-of-avacopan-in-anca-vasculitis-results-from-real-world-data/
