Session Information
Date: Sunday, October 26, 2025
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Citrullinated cathelicidin (LL37), a human-derived antimicrobial peptide, is an autoantigen in psoriatic arthritis (PsA).1 LL37 citrullination by peptidylarginine deiminase (PAD) enzymes attenuates immune activation and downregulates inflammatory responses. PAD2 is the most effective at LL37 citrullination.2 Anti-PAD2 autoantibodies have been described in rheumatoid arthritis3 and Lyme disease4 and are thought to inhibit citrullination of autoantigens such as LL37. This study aimed to identify anti-PAD2 in PsA and to study their association with patient characteristics in a longitudinal PsA registry and biobank.
Methods: Participants in a PsA registry fulfilling CASPAR classification criteria and having banked serum samples were included. We assayed serum anti-PAD2 using an in-house anti-PAD2 ELISA4 in 200 PsA patients and 75 healthy controls (HC). Anti-PAD2 positive cutoff was set at the 90-percentile of HCs. We compared the distribution of patient characteristics in the anti-PAD2 positive and negative groups, using the Wilcoxon rank-sum and Fisher’s Exact tests.
Results: Anti-PAD2 were found in 29% PsA and 9.3% HC sera (p=0.004) (Figure1). 44 (of 58) anti-PAD2 positive and 124 (of 142) anti-PAD2 negative patients had clinical data for analysis. Fifty-nine sera matched with disease activity measured within 48 hours of the serum sample, an additional 9 sera within 6-months, while remaining sera matched with future clinical data (Figure 2). Patients with anti-PAD2 positive compared to negative were older, less likely to have low articular disease activity, and less likely to have active plaque psoriasis affecting the extremities (Table). Considering that 68/168 patients were assessed within 6-months of anti-PAD2 testing, these differences could be attenuated. There were no significant differences in observed treatments.
Conclusion: We discovered anti-PAD2 autoantibodies in PsA. Among PsA patients 29% were positive for anti-PAD2. Anti-PAD2 was associated with more active joint disease and less active skin disease. The clinical significance of anti-PAD2 in PsA needs evaluation in a larger sample using simultaneous measurements with musculoskeletal and skin disease activity.References: 1. Frasca L, et al. Front Immunol. 2018;9:1936. 2. Wong A, et al. The Journal of Immunology. 2018;200(7):2327-2340. 3. Darrah E, et al. Front Immunol.2018;20:9:2696. 4. Kim Y, et al. Front Neurol. 2022;13:874211.
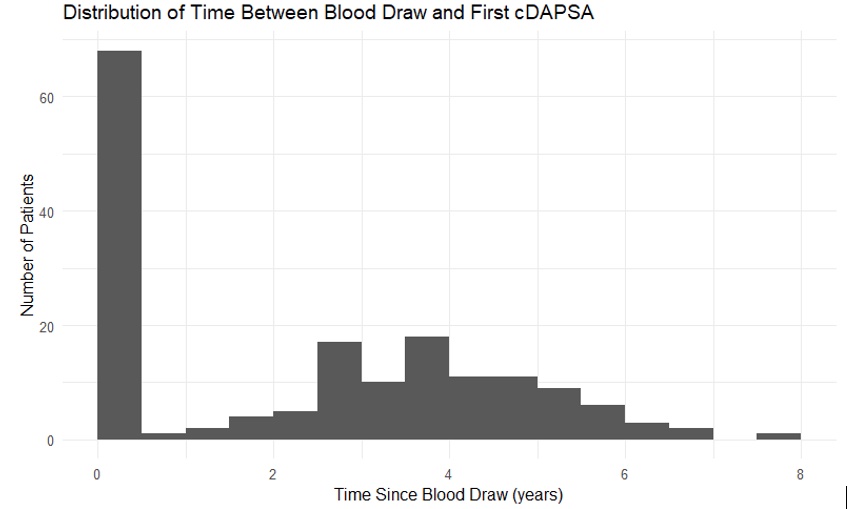 Table. Comparison of distributions of clinical characteristics in anti-PAD2 positive and negative study participants. Numbers and percentages and medians and interquartile ranges are shown. P-values were obtained using the Wilcoxon rank-sum and Fisher’s Exact tests.
Table. Comparison of distributions of clinical characteristics in anti-PAD2 positive and negative study participants. Numbers and percentages and medians and interquartile ranges are shown. P-values were obtained using the Wilcoxon rank-sum and Fisher’s Exact tests.
.jpg) Figure 1. Anti-PAD2 in PsA (n=200) and HCs (n=75) expressed as Arbitrary Units (AU). The red dotted line represents the cut-off value for positivity at 4.393 AU. The mean ± SEM is shown for each group. **Fisher’s Exact test, p = 0.004.
Figure 1. Anti-PAD2 in PsA (n=200) and HCs (n=75) expressed as Arbitrary Units (AU). The red dotted line represents the cut-off value for positivity at 4.393 AU. The mean ± SEM is shown for each group. **Fisher’s Exact test, p = 0.004.
.jpg) Figure 2. Histogram of time between anti-PAD2 testing and clinical assessment: 59 within 48 hours, 9 within 6-months, 100 much later, and 32 samples (not shown) without PsA-specific clinical data.
Figure 2. Histogram of time between anti-PAD2 testing and clinical assessment: 59 within 48 hours, 9 within 6-months, 100 much later, and 32 samples (not shown) without PsA-specific clinical data.
To cite this abstract in AMA style:
Orbai A, Wang H, Grecu M, Meng N, Kim J, Bingham C, Haque U, Miller J, Andrade F, Darrah E, Tiniakou E. Anti-PeptidylArginine Deiminase-2 (anti-PAD2) Autoantibodies in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anti-peptidylarginine-deiminase-2-anti-pad2-autoantibodies-in-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-peptidylarginine-deiminase-2-anti-pad2-autoantibodies-in-psoriatic-arthritis/
