Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Aberrant Neutrophil Extracellular Trap (NET) production contributes to the pathophysiology of multiple inflammatory and autoimmune diseases including rheumatoid arthritis (RA). We report data of a phase 1 trial (EudraCT number:2020-005848-36) with a humanized monoclonal antibody (CIT-013) targeting the citrullinated histones H2A and H4. CIT-013 inhibits NET formation and accelerates the clearance of NETs and netting neutrophils, thus lowering NET tissue burden with significant anti-inflammatory consequences (Chirivi et al 2021; van der Linden et al 2024).
Methods: Prospective, randomized, double-blind, placebo-controlled, phase I trial in participants with RA and healthy volunteers (HV). After obtaining informed consent and completion of baseline assessments, placebo, 25 mg or 50 mg of CIT-013 was administrated subcutaneously at days 1 and 15. Endpoints at day 29 were safety, tolerability, pharmacokinetics (PK) and effect on disease activity as measured with the disease activity score counting 28 joints and CRP (DAS28-CRP).
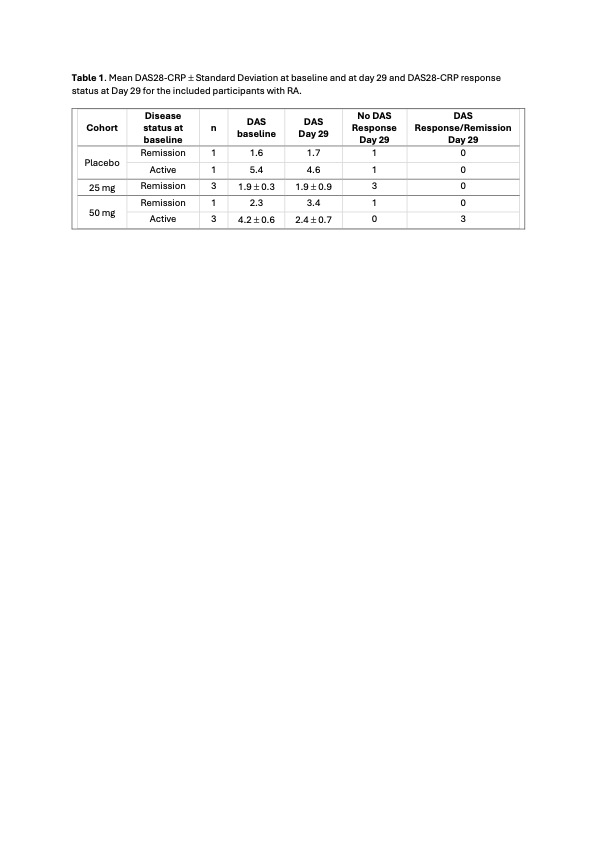
Results: In total 9 participants with RA and 3 HV were dosed with CIT-013, 3 participants received placebo, 3 participants 25 mg CIT-013 and 6 participants 50 mg CIT-013. Two participants with RA received placebo, 3 received 25 mg CIT-013 and 4 received 50 mg CIT-013. All 9 participants with RA met the ACR/EULAR criteria and were on stable synthetic disease modifying anti-rheumatic drugs (sDMARDs).In general, CIT-013 was well tolerated, reported adverse events were mild (grade 1) injection site reactions (all resolved spontaneously) and headache. There was one serious adverse event in a placebo participant with RA that required surgery and hospitalization for a fracture. Anti-drug antibodies were only observed at Day 56 in 2 partcipants with RA, 1 in the 25 mg group and 1 in the 50 mg group, both with low titres (< 4).The half life of CIT-013 was approximately 6 days and all PK parameters were comparable between HV, participants with RA in remission, and participants with RA and active disease.At baseline 4 out of 9 participants with RA (44%) had active disease, defined as a DAS28-CRP of 2.6 or higher. Of these, 1 participant received placebo and continued having active disease, 3 participants received 50 mg CIT-013 and all these had a clinical relevant reduction in disease activity. The clinical activity as observed on day 29 for all 9 participants with RA is captured in Table 1.None of the HV and only 1 RA participant with active disease (8% of total, 25% of participants with active RA) had detectable serum CIT-H3 levels at baseline. In this participant the level dropped below the lowest level of detection (LLD) immediately after the 1st dose.
Conclusion: CIT-013 was well tolerated and a DAS28-CRP reduction was observed in participants with RA and active disease. These findings support further clinical development.
To cite this abstract in AMA style:
Kraan M, Hadi S, Middelink L, Chirivi R, meldrum E, Klarenbeek N, Round P. Phase I Trial in Participants with Rheumatoid Arthritis and Healthy Volunteers with CIT-013, a First in Class NETosis Inhibitor [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/phase-i-trial-in-participants-with-rheumatoid-arthritis-and-healthy-volunteers-with-cit-013-a-first-in-class-netosis-inhibitor/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-i-trial-in-participants-with-rheumatoid-arthritis-and-healthy-volunteers-with-cit-013-a-first-in-class-netosis-inhibitor/