Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: JAKi have been used in rheumatoid arthritis in current practice for several years now, with the progressive arrival on the market of 4 molecules. The use of JAKi has also been hindered by successive safety alerts, which have restricted their use in elderly patients or those with risk factors. The aim of this study was to assess the therapeutic maintenance of the 4 JAKIs available, as well as their efficacy and safety in current RA practice.
Methods: A retrospective study was conducted at a single center, encompassing all RA patients who received a first JAKI between April 2018 and June 2022. The primary endpoint was treatment maintenance at 12 and 24 months, as analyzed using the Kaplan-Meyer survival curve.Secondary endpoints included the efficacy of JAKi and each molecule at 6 months and at the last available visit (until June 2024), as assessed by the DAS28-CRP (Wilcoxon) score, and the number of treatment discontinuations due to inefficacy or intolerance.
Results: We included 108 patients (91 women, 84%) treated with JAKi with a mean age of 57± 14 years and a disease duration of 19± 12 years; rheumatoid factors and ACPA were positive in 91 (84%) and 95 (88%) patients respectively, 83 (77%) had erosions and 37 (34%) extra-articular involvement (Table 1). JAKi were predominantly used in combination with a csDMARD (83/108, 77%) and beyond the 3rd line of targeted therapy (Table 1). The characteristics of patients treated with the 4 JAKi were comparable and are presented in Table 1. Therapeutic maintenance in JAKi was 72% at 12 months and 61% at 24 months. Median treatment survival was 47 months (Figure 1).A significant reduction in DAS28-CRP was observed at 6 months (-1.46± 0.28 vs. inclusion visit, p < 0.001) and at the last visit, which occurred 21± 15 months following JAKi initiation (-1.05± 1.67 vs. inclusion visit, p < 0.001) (Table 2). This decrease in DAS28-CRP appears to be numerically greater with the more selective JAKi (Upadacitinib, Filgotinib) than with Baricitinib and Tofacitinib.During the follow-up period, 45 patients (42%) discontinued JAKi (including 16 patients on Baricitinib, 53%, 6 on Tofacitinib, 60%, 21 on Upadacitinib, 44% and 2 on filgotinib, 10%). The primary reasons for discontinuation included lack of efficacy (26 cases) and intolerance (15 cases). Noteworthy side effects included a myocardial infarction, three pulmonary embolisms, and the detection of breast cancer.
Conclusion: The present study demonstrated that the therapeutic maintenance of JAKi in RA exhibited a relatively high rate of occurrence at 12 and 24 months. Primarily utilized as a third-line targeted therapy, JAKi has been demonstrated to significantly improve RA activity, yielding favorable outcomes. However, a considerable proportion of patients discontinued treatment due to lack of efficacy or adverse effects, underscoring the necessity for ongoing, personalized evaluation of these therapies in routine clinical practice.
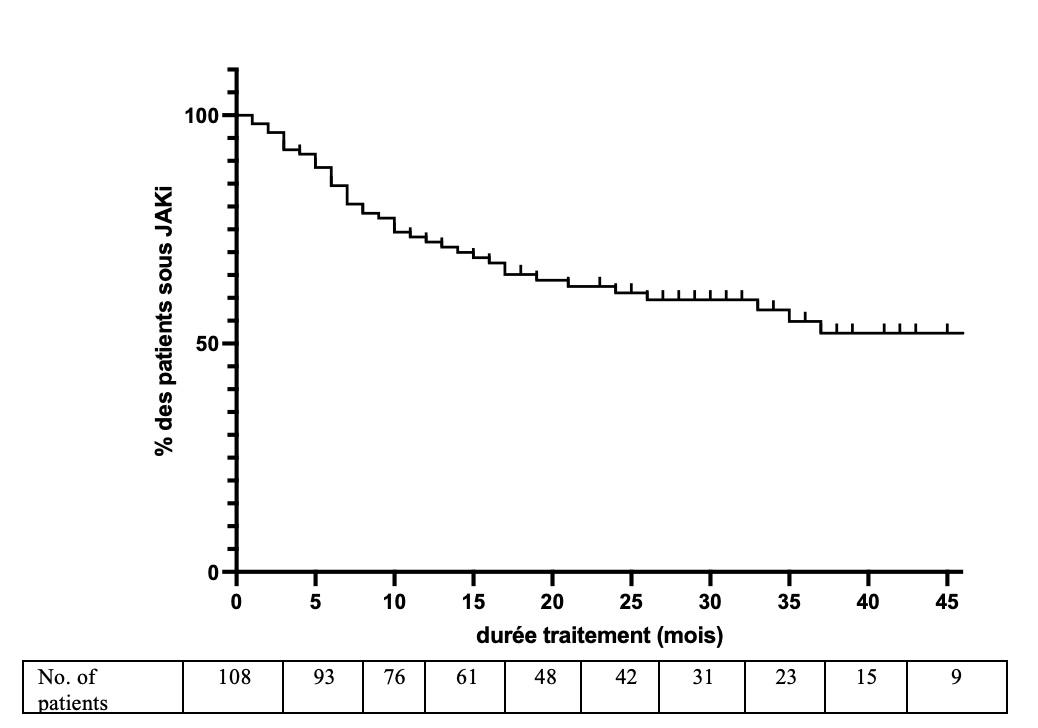 Table 1: Characteristics of patients treated with JAKi
Table 1: Characteristics of patients treated with JAKi
.jpg) Table 2: Efficacy of JAKi on DAS28-CRP
Table 2: Efficacy of JAKi on DAS28-CRP
.jpg) Figure 1: Therapeutic maintenance of JAKi
Figure 1: Therapeutic maintenance of JAKi
To cite this abstract in AMA style:
Al Tabaa O, Abdellaoui S, Hecquet S, Thomas M, Carves S, Combier A, Fogel O, Allanore Y, Avouac J. Efficacy and safety profile of JAK inhibitors in current practice in rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-profile-of-jak-inhibitors-in-current-practice-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-profile-of-jak-inhibitors-in-current-practice-in-rheumatoid-arthritis/
