Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The efficacy and safety of sarilumab (SAR) and methotrexate (MTX) in patients (pts) in Japan with moderate-to-severely active rheumatoid arthritis (RA) and inadequate response to MTX have been demonstrated in the phase 3 KAKEHASI trial (NCT02293902). This post hoc analysis evaluated the efficacy of sarilumab in pts with RA by disease activity at treatment initiation.
Methods: In the KAKEHASI study of SAR with MTX, pts were randomized 2:2:1:1 to receive SAR 150 or SAR 200 mg every 2 wks (q2w) for 52 weeks (wks), or placebo for 24 wks followed by SAR 150 or SAR 200 mg q2w for 28 wks. Here, pts from the efficacy analysis population were stratified by Clinical Disease Activity Index (CDAI) scores at SAR initiation (baseline [BL]) as: low disease activity (BL-LDA), CDAI >2.8 and ≤10; moderate disease activity (BL-MDA), CDAI >10 and ≤22; and high disease activity (BL-HDA), CDAI >22. Differences in baseline characteristics and SAR response measured by CDAI, DAS28-CRP, American College of Rheumatology [ACR]20/50/70, HAQ-DI and visual assessment score [VAS]) were assessed. Pts with high disease activity were further stratified into two (LHDA: >22 and ≤35.7; HHDA: >35.7) and four subgroups (HDA1, CDAI >22 and ≤29.5; HDA2, CDAI >29.5 and ≤35.7; HDA3, CDAI >35.7 and ≤43.3; and HDA4, CDAI >43.3).
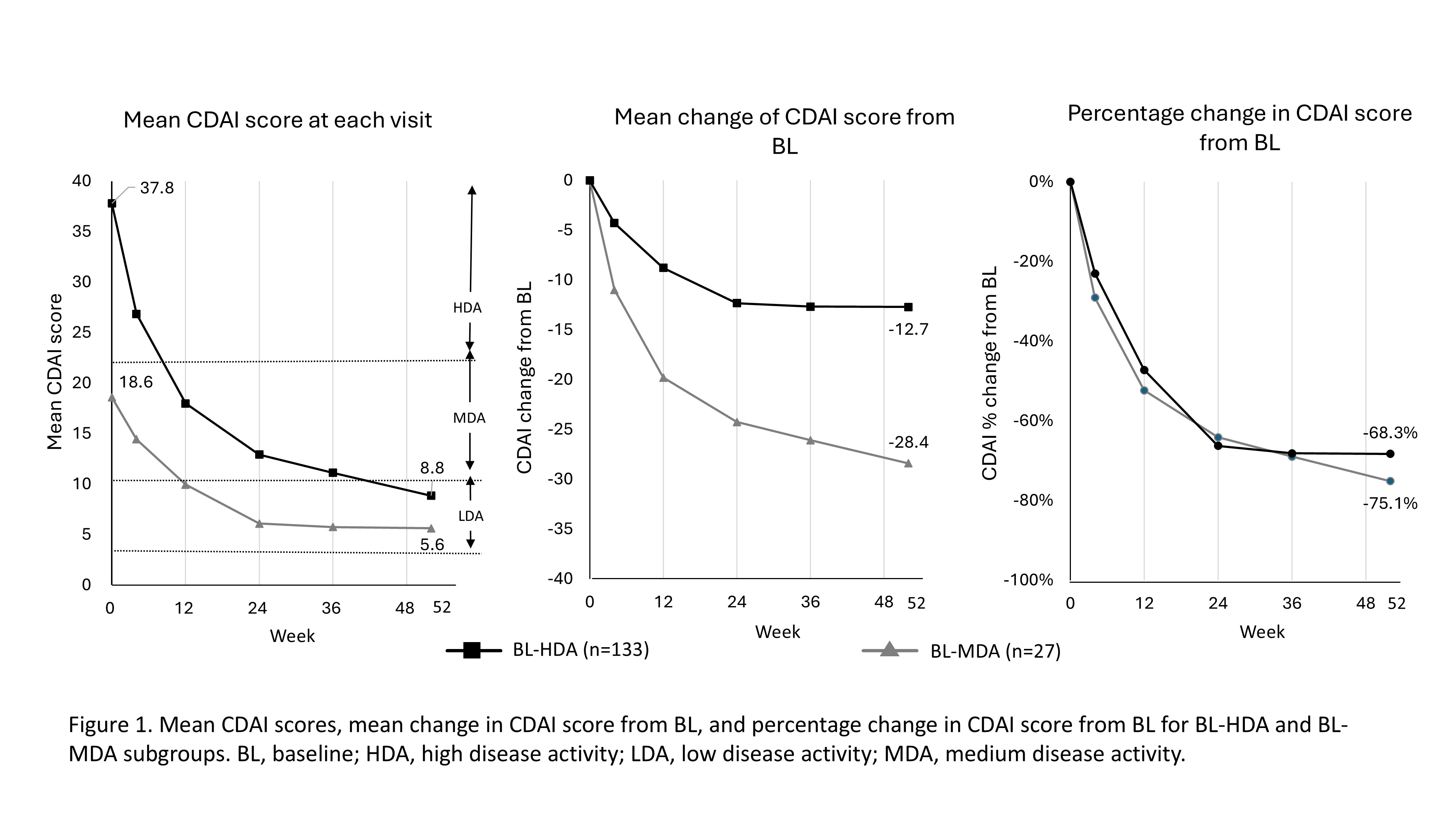
Results: In total, 243 pts (female, 76.3–81%; mean age, 51.9–56.1 years) were randomized to receive SAR 150 mg q2w (n=81), SAR 200 mg q2w (n=80), or placebo followed by SAR 150 mg q2w (n=42) or SAR 200 mg q2w (n=40). The pooled efficacy analysis population (n=242) was stratified as: BL-HDA, n=204 (84.3%), BL-MDA, n=37 (15.3%), and BL-LDA, n=1 ( < 1%). BL characteristics were similar between BL-MDA and BL-HDA groups (female, 73.0% and 78.5%; ≥65 years, 81.1% and 77.6%, respectively). Mean CDAI scores at BL for BL-HDA and BL-MDA groups were 37.8 (n=133) and 18.6 (n=27), decreasing to 8.8 (n=105) and 5.6 (n=17), respectively, by 52 wk (Figure 1). Change in mean (%) CDAI scores over 52 wks was similar between groups (BL-HDA, −28.4 [−75.1%]; BL-MDA, −12.7 [−68.3%]). Of all SAR-treated pts, >50% achieved LDA or remission at 52 wks (BL-HDA, 56.4% [75/133]; BL-MDA, 55.6% [15/27]). The trends in changes of mean (%) CDAI score were also reflected in those of DAS28-CRP scores (BL-MDA, −2.2 [−51.0%]; BL-HDA, −3.4 [−58.7%]) and HAQ-DI scores (BL-MDA, −0.41 [−48.6%]; BL-HDA, −0.67 [−54.2%]). Physician Global VAS also followed this trend for the BL-MDA (−34.5 [−76.0%]) and BL-HDA (−49.7 [−77.5%]) groups. Incidence of ACR20/50/70 response was similar regardless of BL-CDAI activity (HDA/MDA), but slightly higher in the BL-HDA group (Figure 2). In the two-subgroups, although the mean change of CDAI from BL at 52 wks was higher in the HHDA (−36.7) than the LHDA group (−21.4), the percentage change was notably similar (−72.9% and −79.1%, respectively), as per the HDA/MDA groups. In the four-subgroups, pts in HDA-3 and HDA-4 subgroups with the highest disease activity had a greater absolute decrease of CDAI than other groups with a similar percentage change, yet no plateau by 52 wks (Figure 3).
Conclusion: These results indicate that the efficacy of SAR is generally similar across pts with RA of different disease activity.
To cite this abstract in AMA style:
Kameda H, Takahashi T, Soeda N, Tanaka Y. Efficacy of sarilumab in patients with rheumatoid arthritis of different disease activity at treatment initiation: a post-hoc analysis of a Phase 3 trial of sarilumab in Japan [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-sarilumab-in-patients-with-rheumatoid-arthritis-of-different-disease-activity-at-treatment-initiation-a-post-hoc-analysis-of-a-phase-3-trial-of-sarilumab-in-japan/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-sarilumab-in-patients-with-rheumatoid-arthritis-of-different-disease-activity-at-treatment-initiation-a-post-hoc-analysis-of-a-phase-3-trial-of-sarilumab-in-japan/

.jpg)
.jpg)