Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumathoid Arthritis (RA) is a chronic inflammatory disease that affects the musculoskeletal system. Early and effective treatment is important to improve the quality of life of patients and prevent irreversible damage. Upadacitinib (UPA) is a Janus Kinase inhibitor (JAKi) selective for JAK1, approved for RA in 2019. In the Basque Country, since May 2022, UPA has been the prioritized after Adalimumab biosimilar failure in RA patients. The aim of this study was to evaluate the efficacy of UPA in RA patients treated with JAKi previously and to describe the characteristics of the patients and clinical response.
Methods: 7 hospitals in our region (1.5M inhabitants) participated in a multicenter, observational and ambispective study that included patients with UPA prescription from June 2021 to May 2024, with follow-up until Dec 2024. In our study, 248 patients were divided into two groups based on who received a previous JAKi treatment or not. We used SDAI to measure the disease activity at six visits.
Results: Of the 248 RA patients included, 84.1% were women, the mean age was 55.9 years (SD 12.30). The rest of the baseline characteristics are reflected in Table 1. The first group contained 91 patients who received JAKi previously (JAKi group), 94.5% Baricitinib suspended for lack of efficacy. The other group containing 157 patients did not receive JAKi before the treatment (No JAKi group). When both groups were compared, the JAKi group showed a higher refractory rate (55% needed two or more biologicals compared to 17%) and a longer disease duration (140 months vs 107 months). At the beginning of the treatment with UPA, 91.1% (226) of the patients had a high/moderate activity (SDAI) with no difference between both groups (91.2% vs 90.4%). As shown in Figure 1, the No JAKi group achieved a higher percentage of patients with a low activity and remission. However, these differences did not reach statistical significance. Both groups showed similar trends regarding treatment continuation and reasons for discontinuation with inefficiency as the primary cause in the later visits. Most adverse effects appeared in the first 3 months. Although JAKi showed a lower suspension rate at 3 months, there were no statistically significant differences. Median UPA persistence in RA was 22.71 months (SE 1.67148 and 95% CI 18.5733), slightly shorter for the JAKI group (22.02 months; SE 1.6571 and 95% CI 18.2774), but not reaching statistical significance. No differences were found in terms of adherence to the treatment in the first two years (91% vs 93%).
Conclusion: In our study, the JAKi-experienced group showed no significant differences in terms of patient characteristics, comorbidities, baseline disease activity, or co-treatment with Prednisone and csDMARDs. Only the disease duration and refractoriness to previous biologic treatments were significantly higher in the JAKi-experienced group. Clinical response and drug persistence (median almost 2 years) were good and comparable between both groups, as were discontinuation rates and causes (inefficacy). Therefore, in our study, UPA appears to be a good treatment option for RA patients who are refractory and have been previously treated with Baricitinib.
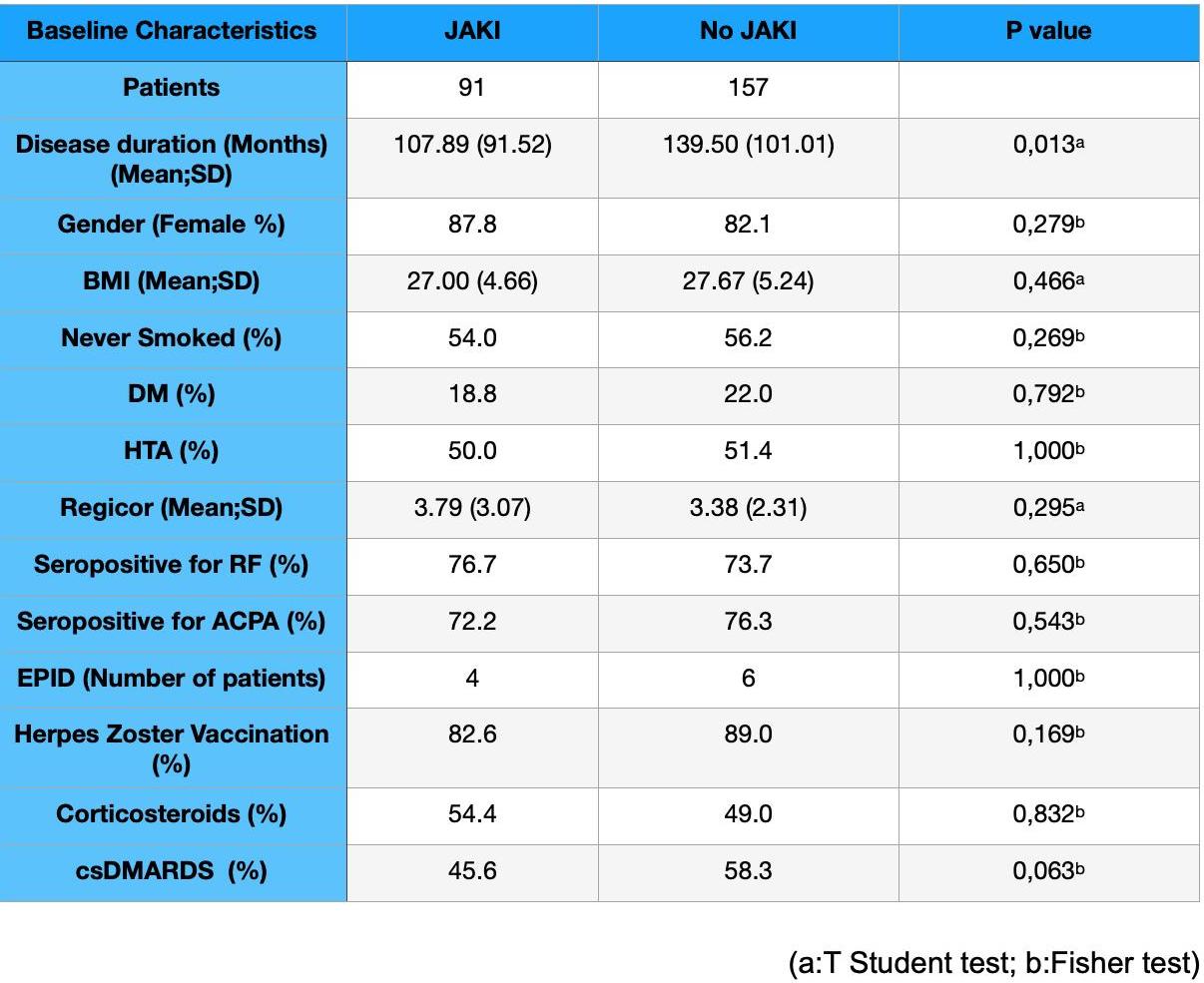 Table 1. Patients’ baseline characteristics.
Table 1. Patients’ baseline characteristics.
.jpg) Figure 1. Comparative activity levels over time by group.
Figure 1. Comparative activity levels over time by group.
To cite this abstract in AMA style:
Gonzalez Mozo de Rosales G, Lopez-Dominguez L, Alcorta-Lorenzo N, ibarguengoitia Barrena o, Montero D, Ruibal Escribano A, barastay Alberdi e, Valero Jaimes J, Ibarrola L, Garcia Escudero P, Lopez I Gomez M, Galindez Aguirregoicoa E, Garcia Vivar M. Multicenter study on the use of Upadacitinib: Results in RA patients previously treated with Baricitinib [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/multicenter-study-on-the-use-of-upadacitinib-results-in-ra-patients-previously-treated-with-baricitinib/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multicenter-study-on-the-use-of-upadacitinib-results-in-ra-patients-previously-treated-with-baricitinib/
