Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: In April 2022, the Canadian province of Quebec introduced a mandatory non-medical switch to biosimilars for persons on bio-originators to manage healthcare costs. Our objective is to describe real-world experience with biosimilars used for inflammatory arthritis in the context of this policy change.
Methods: We used data from three adult clinical registries in Quebec: the RHUMADATA prospective arthritis registry, the Early Undifferentiated Polyarthritis (EUPA) cohort, and the University of Sherbrooke Registry of Advanced Therapies (USRAT). We studied individuals who transitioned from the originator biologics to their equivalent biosimilars – infliximab, etanercept (ETA), adalimumab (ADA), and rituximab– from the time of the non-medical switching policy (April 2021) through May 2023. The study period began one year before the implementation of the non-medical switching policy to capture the transition phase leading up to and following the policy’s introduction.Baseline characteristics were assessed at the time of biosimilar initiation, including age, sex, underlying condition (ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis), disease duration, body mass index, alcohol and tobacco use, and quality of life (Health Assessment Questionnaire for RHUMADATA, and the Modified HAQ for EUPA and USRAT). Prior medication history, including other biologics, corticosteroids, and non-biologic immunosuppressants, was also reviewed. Reasons for discontinuation were documented by treating physicians. Discontinuation was estimated using Kaplan-Meier methods.
Results: We studied 110 individuals with inflammatory arthritis, most switching to ADA (68%) or ETA (20%) biosimilars. Females were 61% of the cohort, and at biosimilar transition, the mean age was 56.9 years (standard deviation 15.8). Additional baseline characteristics are presented in Table 1. Individuals were followed for a median of 1.5 years (interquartile range 0.5 to 1.9 years). During this time, 16 individuals discontinued their treatment, for a discontinuation rate of 115.7 (95% confidence interval, CI 66.1-187.8) events per 1,000 person-years. As seen in Figure 1, the probability of discontinuation was 11.2% (95% CI: 4.4%-17.6%) by Year 1 and 18.2% % (95% CI: 9.3% to 26.2%) by Year 2. The most common reason for discontinuation was treatment failure in 7 individuals.
Conclusion: Among individuals with arthritis who underwent mandatory non-medical switching from bio-originators to biosimilars, about 80% remain on the biosimilar at 2 years. Thus, discontinuation rates appear consistent with those observed among experienced biologic users, suggesting relative stability despite the policy-driven switch.
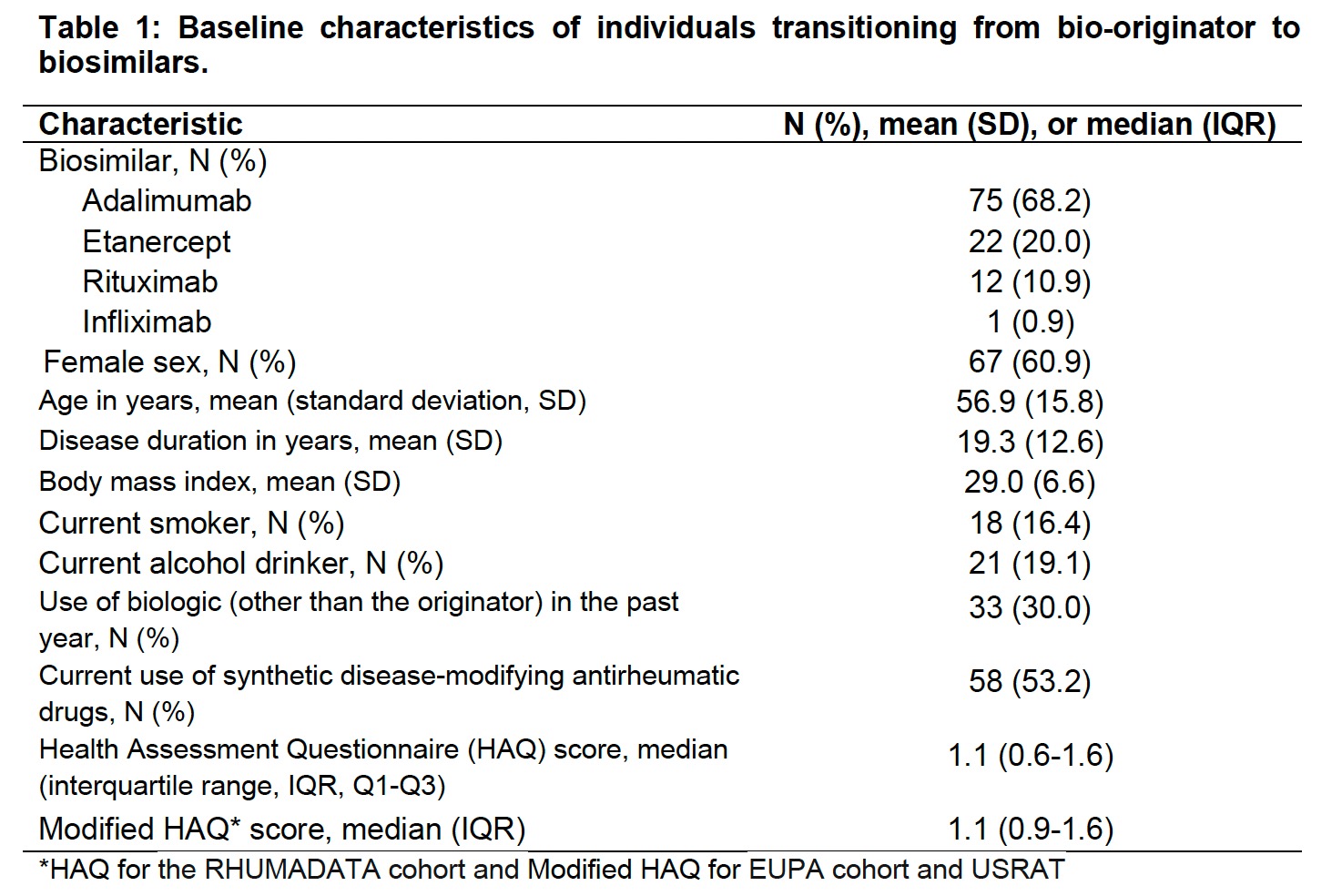 Table 1: Baseline characteristics of individuals transitioning from bio-originator to biosimilars
Table 1: Baseline characteristics of individuals transitioning from bio-originator to biosimilars
.jpg) Figure 1. Kaplan-Meier survival curves showing the probability of discontinuing the biosimilar therapy over follow-up, with shaded areas representing 95% confidence intervals (95% CI).
Figure 1. Kaplan-Meier survival curves showing the probability of discontinuing the biosimilar therapy over follow-up, with shaded areas representing 95% confidence intervals (95% CI).
To cite this abstract in AMA style:
Moura C, Lukusa L, Choquette D, Boire G, Carrier N, Neville A, Bernatsky S. Drug Discontinuation in Inflammatory Arthritis Following Mandatory Non-Medical Switching from Originator to Biosimilar in Quebec, Canada [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/drug-discontinuation-in-inflammatory-arthritis-following-mandatory-non-medical-switching-from-originator-to-biosimilar-in-quebec-canada/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-discontinuation-in-inflammatory-arthritis-following-mandatory-non-medical-switching-from-originator-to-biosimilar-in-quebec-canada/
