Session Information
Date: Sunday, October 26, 2025
Title: (0430–0469) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is a potentially life-threatening extra-articular manifestation of RA. The comparative safety of disease-modifying antirheumatic drugs (DMARDs) in RA patients, particularly those with coexisting ILD, remains unclear. We aimed to assess the risk of ILD development and serious adverse events across DMARD classes using a nationwide claims database.
Methods: We conducted a nationwide cohort study using the Korean National Health Insurance Service database (2011–2020). DMARD treatment episodes were analyzed in RA patients aged ≥19 years without baseline ILD to assess the risk of incident ILD. In RA-ILD patients, treatment episodes initiated after ILD diagnosis were used to evaluate safety outcomes. A detailed flowchart of patient selection and episode classification is shown in Figure 1. DMARD exposure was categorized into four groups: conventional synthetic DMARDs (csDMARDs), tumor necrosis factor inhibitors (TNFi), non-TNF biologic agents (non-TNFi), and Janus kinase inhibitors (JAKi). Drug exposure was modeled as time-varying, and adjusted hazard ratios (aHRs) were estimated using IPTW-weighted Cox regression.
Results: Among 209,852 treatment episodes in RA patients without baseline ILD, the overall incidence rate of ILD was 3.19 per 1,000 person-years. In time-varying IPTW-adjusted Cox models, none of the DMARD classes were significantly associated with ILD development compared to csDMARDs (non-TNFi aHR 1.51, 95% CI 0.91–2.50; JAKi aHR 0.94, 95% CI 0.44–2.02; TNFi aHR 0.89, 95% CI 0.51–1.56). Among 1,688 treatment episodes in RA-ILD patients, non-TNF biologics were associated with significantly increased risks of all-cause hospitalization (aHR 1.35, 95% CI 1.02–1.80), infection (aHR 1.51, 95% CI 1.06–2.15), and death (aHR 4.31, 95% CI 2.21–8.41) compared to csDMARDs (Table 1). Non-TNFi use was also associated with a higher risk of infection recurrence (aHR 1.35, 95% CI 1.01–1.79) and pneumonia-related hospitalization (aHR 1.90, 95% CI 0.98–3.69). In contrast, hospitalization due to ILD exacerbation did not differ significantly across treatment groups. The risk of major adverse cardiovascular events was also comparable between groups.
Conclusion: In RA patients without baseline ILD, the risk of developing ILD did not significantly differ across DMARD classes. In RA-ILD patients, while rates of hospitalization due to ILD exacerbation were comparable across groups, non-TNFi use was associated with increased risks of infection and pneumonia-related outcomes. Given that pneumonia is a leading cause of death in RA-ILD, close infection surveillance and preventive strategies are warranted when using non-TNF biologics in this population.
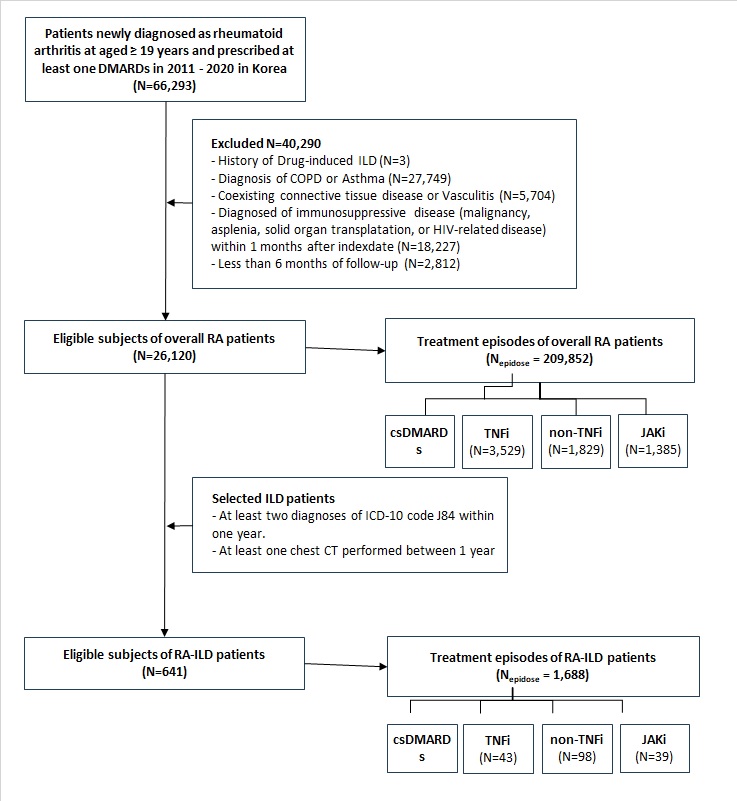 Figure 1. Study design and selection of treatment episodes in RA and RA-ILD cohorts.
Figure 1. Study design and selection of treatment episodes in RA and RA-ILD cohorts.
Flowchart showing the selection of eligible RA patients (Nf26,120) and RA-associated ILD (RA-ILD) patients (Nf641) from the Korean National Health Insurance Service database (2011–2020), along with the number of treatment episodes analyzed for each DMARD category in both cohorts.
.jpg) Table 1. Incidence and Adjusted Risks of Adverse Events by DMARD Class in RA-ILD Patients
Table 1. Incidence and Adjusted Risks of Adverse Events by DMARD Class in RA-ILD Patients
To cite this abstract in AMA style:
Lee k, Lee B, Kim H, Kim S. Safety of DMARDs in Rheumatoid Arthritis: A Nationwide Study of ILD Risk and Outcomes in RA-ILD [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-of-dmards-in-rheumatoid-arthritis-a-nationwide-study-of-ild-risk-and-outcomes-in-ra-ild/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-dmards-in-rheumatoid-arthritis-a-nationwide-study-of-ild-risk-and-outcomes-in-ra-ild/
