Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
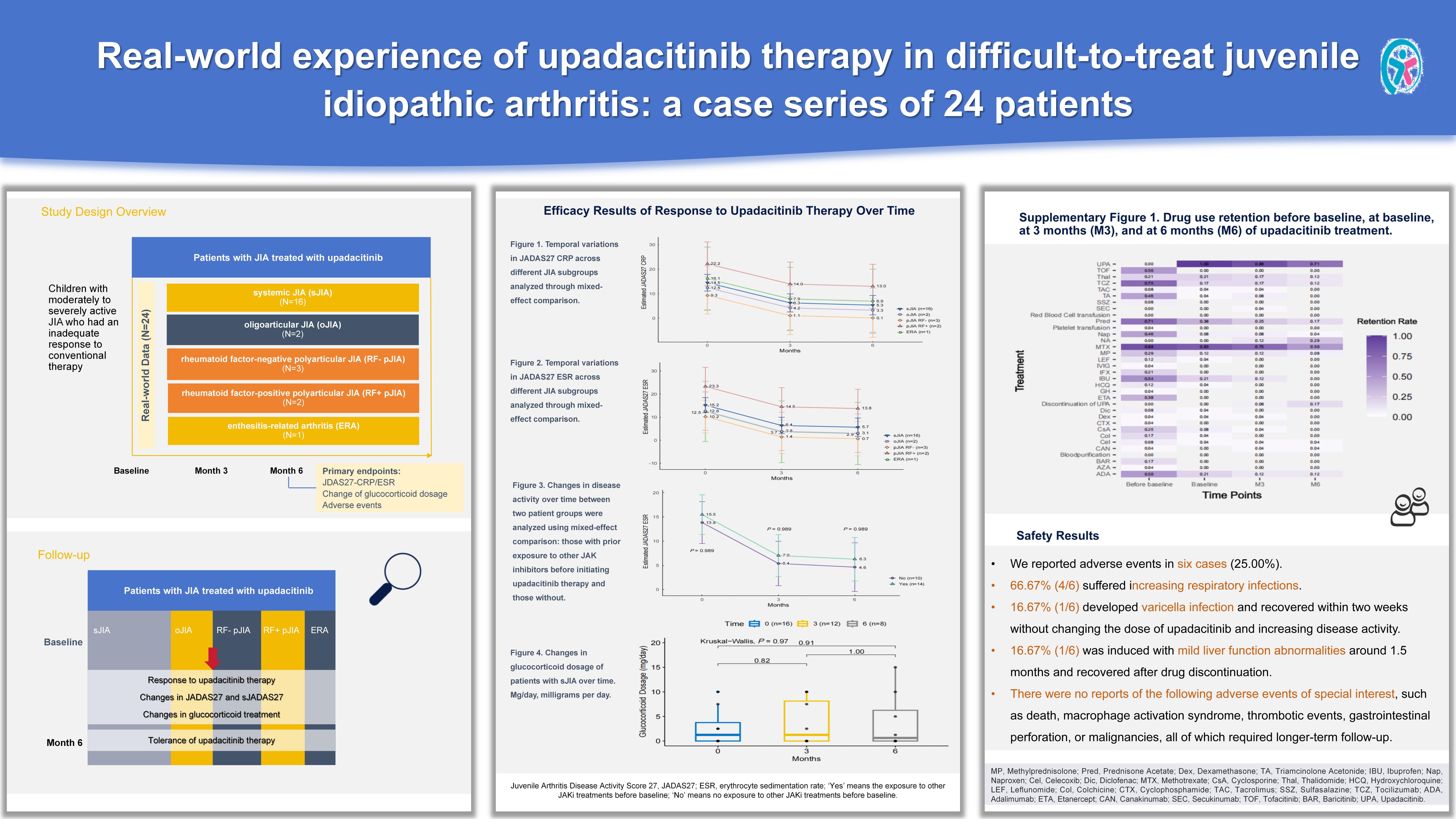
Background/Purpose: Juvenile idiopathic arthritis (JIA) is a chronic rheumatic disease. Some patients experiencing active disease due to inadequate responses to conventional treatments. Studies have demonstrated effectiveness of JAK inhibitors (JAKis) among JIA patients. This study aimed to evaluate the efficacy and safety of upadacitinib in JIA patients.
Methods: We included 24 difficult-to-treat JIA patients in China treated with upadacitinib from January 1, 2024, to December 31, 2024. Juvenile arthritis disease activity scores (JADAS) and efficacy outcomes were collected. Differences between groups were analyzed using univariable and mixed-effect model analyses. A two-sided P value < 0.05 was considered statistically significant in this study.
Results: Total patients received upadacitinib for a median duration of 7.00 (range: 3.50-10.50) months. Baseline median JADAS27 CRP and JADAS27 ESR were 14.59 (range: 8.00-18.61) and 15.05 (range: 12.50-19.60), respectively. Both scores significantly decreased to 3.75 (range: 2.00, 10.00) and 3.75 (range: 2.25, 7.85) at 3 months and sustained improvements at 6 months (P < 0.001). Median prednisone dose decreased from 1.88 (range: 0.00, 7.50) mg/day to 0.63 (range: 0.00, 7.50) mg/day at 3 months and 0.00 (range: 0.00, 5.00) mg/day at 6 months. Tolerance was acceptable with several cases of elevated transaminases and increased upper respiratory tract infections.
Conclusion: This is the largest real-world study of upadacitinib in JIA to date and provides encouraging evidence of the drug’s efficacy and safety. The study also indicates that irrespective of prior treatment with other JAKis, switching to upadacitinib may help regain control of disease activity in patients with difficult-to-treat JIA.
 Graphical Abstract Image of Real-world experience of upadacitinib therapy in difficult-to-treat juvenile idiopathic arthritis a case series of 24 patients.
Graphical Abstract Image of Real-world experience of upadacitinib therapy in difficult-to-treat juvenile idiopathic arthritis a case series of 24 patients.
Figure 1. Temporal variations in JADAS27 CRP across different JIA subgroups analyzed through mixed-effect comparison. Juvenile Arthritis Disease Activity Score 27, JADAS27; CRP, C-reactive protein.
Figure 2. Temporal variations in JADAS27 ESR across different JIA subgroups analyzed through mixed-effect comparison. Juvenile Arthritis Disease Activity Score 27, JADAS27; ESR, erythrocyte sedimentation rate.
Figure 3. Changes in disease activity over time between two patient groups were analyzed using mixed-effect comparison: those with prior exposure to other JAK inhibitors before initiating upadacitinib therapy and those without. Juvenile Arthritis Disease Activity Score 27, JADAS27; ESR, erythrocyte sedimentation rate; ‘Yes’ means the exposure to other JAKi treatments before baseline; ‘No’ means no exposure to other JAKi treatments before baseline.
Figure 4. Changes in glucocorticoid dosage of patients with sJIA over time.
Mg/day, milligrams per day.
Supplementary Figure 1. Drug use retention before baseline, at baseline, at 3 months (M3), and at 6 months (M6) of upadacitinib treatment. MP, Methylprednisolone; Pred, Prednisone Acetate; Dex, Dexamethasone; TA, Triamcinolone Acetonide; IBU, Ibuprofen; Nap, Naproxen; Cel, Celecoxib; Dic, Diclofenac; MTX, Methotrexate; CsA, Cyclosporine; Thal, Thalidomide; HCQ, Hydroxychloroquine; LEF, Leflunomide; Col, Colchicine; CTX, Cyclophosphamide; TAC, Tacrolimus; SSZ, Sulfasalazine; TCZ, Tocilizumab; ADA, Adalimumab; ETA, Etanercept; CAN, Canakinumab; SEC, Secukinumab; TOF, Tofacitinib; BAR, Baricitinib; UPA, Upadacitinib.
To cite this abstract in AMA style:
Tang X, Luo X, Luo X, Ding M, Yang X, Liu D, Xu L, Zhang Z, An Y, Zhao X. Real-world experience of upadacitinib therapy in difficult-to-treat juvenile idiopathic arthritis: a case series of 24 patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-experience-of-upadacitinib-therapy-in-difficult-to-treat-juvenile-idiopathic-arthritis-a-case-series-of-24-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-experience-of-upadacitinib-therapy-in-difficult-to-treat-juvenile-idiopathic-arthritis-a-case-series-of-24-patients/
