Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Secukinumab demonstrated efficacy and safety in patients with juvenile idiopathic arthritis (JIA), including enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA), for up to 2 years in the JUNIPERA study.1 The JUNIPERA clinical program included an extension study to assess the long-term efficacy and safety of secukinumab in patients with ERA and JPsA. Here we report the 4-year efficacy and safety results of the long-term extension (LTE) study.
Methods: Of 86 patients in the primary study, 55 deemed by the investigator to benefit from secukinumab therapy opted to enter the LTE study. Of these, 54 received secukinumab treatment (subcutaneous; 75/150 mg in patients < 50/≥50 kg) every 4 weeks up to 4 years. Secukinumab dose could be escalated from 75 to 150 mg or 150 to 300 mg, based on the investigator judgment of disease activity. Efficacy outcomes were presented as observed for JIA American College of Rheumatology (JIA ACR) 30/50/70/90/100 responses, inactive disease (ID) status over time (from weeks 104 to up to 312), and change from baseline in Juvenile Arthritis Disease Activity Score (JADAS-27 and JADAS-71) along with adverse events (AEs).
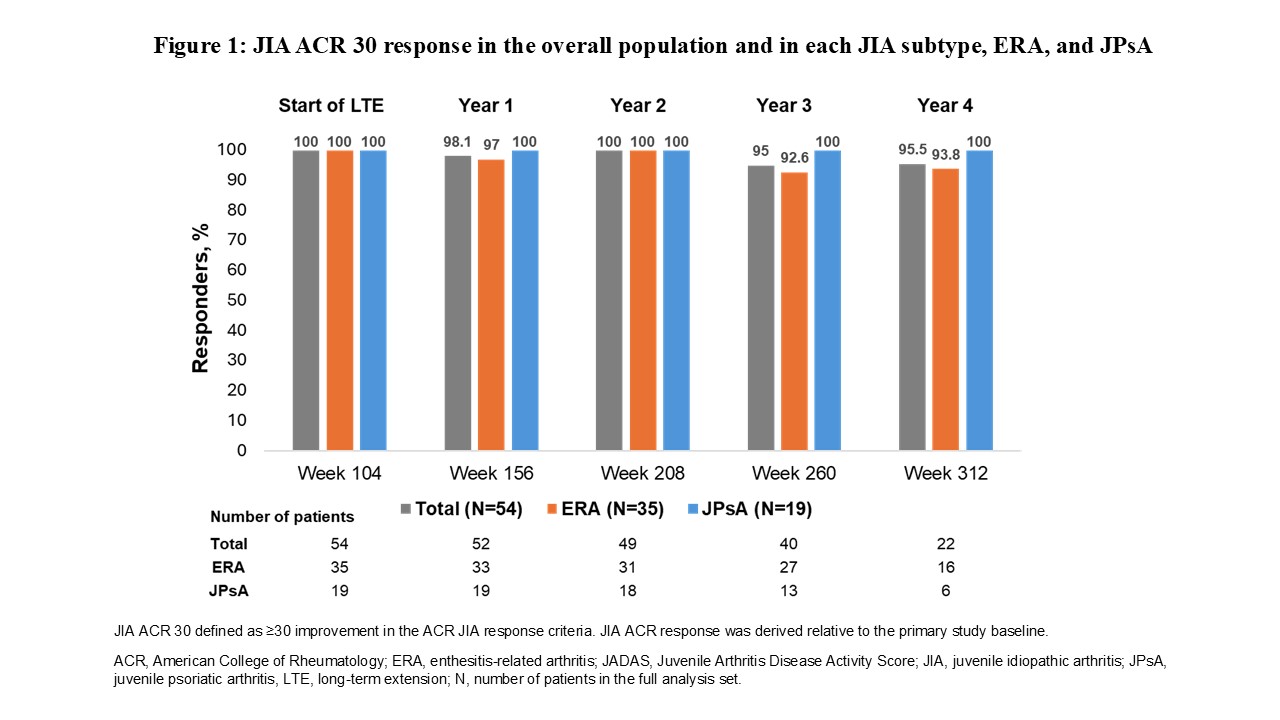
Results: The mean (SD) age of the overall JIA population was 12.5 (3.4) years, and 66.7% were male. A total of 22 patients (40%) underwent dose escalation. Most patients achieved JIA ACR 30 responses ( >93%) at all visits during the LTE (Figure 1). The JIA ACR 50/70/90/100 (Figure 2), JADAS-27 and JADAS-71 responses (Figure 3), as well as the ID status achieved during the primary study, were maintained in the overall population and the ERA and JPsA subtypes in the LTE. Of note, the JIA ACR complete response (JIA ACR 100) was achieved by ≥50% of patients at all visits during the LTE.With 182.2 patient-years of secukinumab exposure during the LTE, 46 patients (85.2%) experienced at least one AE. Most AEs were considered not related to the study treatment and were generally mild (n=25, 46.3%) to moderate (n=17, 31.5%) in severity. A total of 9 non-fatal serious AEs (SAEs) were experienced by 4 (7.4%) patients, and except for 1 event of Crohn’s disease (1.9%) that led to treatment discontinuation, all SAEs were considered not related to secukinumab treatment and were recovered/resolved during study participation. The most common treatment-emergent AEs by system organ class were infections and infestations (n=33, 61.1%). No deaths were reported during the LTE period or during primary study.
Conclusion: Long-term sustained clinical benefits of secukinumab were observed in patients with JIA (ERA and JPsA subtypes) for up to 4 years of exposure in the LTE study, along with a favorable long-term safety profile. Reference:Brunner HI, et al. Ann Rheum Dis. 2023;82(1):154-160.
To cite this abstract in AMA style:
Brunner H, Foeldvari I, Horneff G, Mera Varela A, Ravelli A, Kaur S, Suhas Dahale S, Martin R, Lovell D, Martini A, Ruperto N. Juvenile Psoriatic Arthritis and Enthesitis-Related Arthritis: 4-Year Results From the JUNIPERA Extension Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/juvenile-psoriatic-arthritis-and-enthesitis-related-arthritis-4-year-results-from-the-junipera-extension-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/juvenile-psoriatic-arthritis-and-enthesitis-related-arthritis-4-year-results-from-the-junipera-extension-study/

.jpg)
.jpg)