Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Juvenile idiopathic arthritis (JIA), the most common pediatric rheumatic disease, has been tied to microbiome disruption (dysbiosis). Dysbiosis in adults with arthritis affects response to methotrexate, a common disease-modifying antirheumatic drug (DMARD), but the effects of microbiome-altering drugs (e.g., antibiotics) on methotrexate response for JIA are unknown. We tested if antibiotic exposure is associated with response to methotrexate for JIA, hypothesizing shorter times to treatment change after antibiotic exposure and larger effects with more recent, frequent, and disruptive exposures.
Methods: We conducted a retrospective cohort study using national US public insurance administrative claims data (2001-2019). We included children ages 1-17 diagnosed with JIA who were initiating methotrexate monotherapy or, for comparison, tumor necrosis factor inhibitor (TNFi) monotherapy, after ≥10 months of continuous enrollment without DMARDs. Antibiotic exposure during the 10-month baseline period was assessed and characterized by timing, number of courses, and type. Nonbacterial antimicrobial drugs were evaluated as a negative control exposure. As a proxy of treatment ineffectiveness, the primary outcome was initiation of a second DMARD after at least 1 month, a lag representing hypothetical induction time between antibiotic-induced dysbiosis and altered DMARD response, allowing for delayed initiation of a DMARD intended for use in combination. Censoring occurred after a 60-day gap in DMARDs, completion of 10 months of follow-up, loss of enrollment, or death. Associations between antibiotic exposure and treatment change were estimated using Cox regression, adjusting for baseline demographic, disease, treatment, and health utilization covariates (Table), and represented by adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). In sensitivity analyses, we considered outcomes occurring after a 2-month lag after initial DMARD exposure.
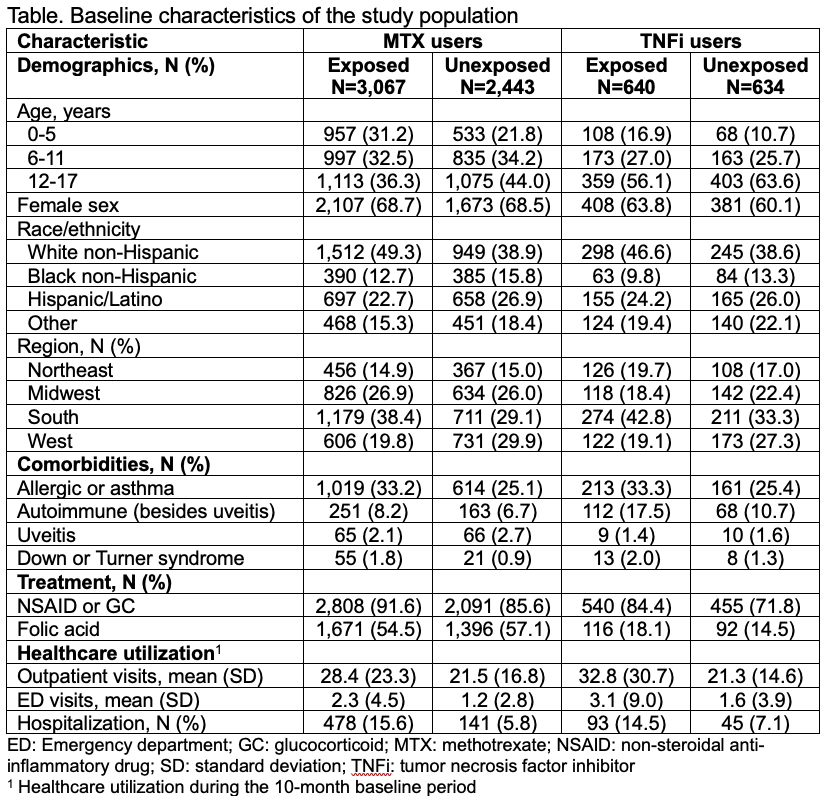
Results: We identified 5510 new methotrexate users (mean age 9.5±4.9 years, 68.6% female, 55.6% exposed to antibiotics, 15.9% with outcomes) and 1274 new TNFi users (mean age 11.6±4.5 years, 61.9% female, 50.2% exposed to antibiotics, 15.6% with outcomes). Children with antibiotic exposure tended to be younger and more likely to be White non-Hispanic, live in the South, and have more comorbidities, more anti-inflammatory exposure, and more baseline healthcare utilization (Table). No relationship was observed between baseline antibiotic exposure and treatment change in methotrexate users (HR 0.98, 95% CI 0.85, 1.13) or TNFi users (HR 1.13, 95% CI 0.83, 1.53). Antibiotic timing, number of courses, and type of antibiotic exposure, as well as nonbacterial antimicrobial drug exposure, were also not associated with treatment changes in methotrexate or TNFi users (Figures 1-2). Findings from sensitivity analyses were consistent.
Conclusion: Recent antibiotic exposure is not associated with changes in DMARD treatment in children with JIA, suggesting that dysbiosis does not play a major role in response to methotrexate or TNFi for JIA.
.jpg) Figure 1. Associations between antibiotic exposure and MTX ineffectiveness. Forest plot shows associations between antibiotic exposure within 10-months before methotrexate (MTX) initiation and MTX ineffectiveness, as represented by initiation of a second DMARD after ≥1 month. Models were adjusted for baseline demographic, clinical, treatment, and healthcare-related covariates (see Table) and expressed as adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). HRs >1 signify shorter times to initiation of a second DMARD with antibiotic exposure.
Figure 1. Associations between antibiotic exposure and MTX ineffectiveness. Forest plot shows associations between antibiotic exposure within 10-months before methotrexate (MTX) initiation and MTX ineffectiveness, as represented by initiation of a second DMARD after ≥1 month. Models were adjusted for baseline demographic, clinical, treatment, and healthcare-related covariates (see Table) and expressed as adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). HRs >1 signify shorter times to initiation of a second DMARD with antibiotic exposure.
.jpg) Figure 2. Associations between antibiotic exposure and TNFi ineffectiveness. Forest plot shows associations between antibiotic exposure within 10-months before tumor necrosis factor inhibitor (TNFi) initiation and TNFi ineffectiveness, as represented by initiation of a second DMARD after ≥1 month. Models were adjusted for baseline demographic, clinical, treatment, and healthcare-related covariates (see Table) and expressed as adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). HRs >1 signify shorter times to initiation of a second DMARD with antibiotic exposure.
Figure 2. Associations between antibiotic exposure and TNFi ineffectiveness. Forest plot shows associations between antibiotic exposure within 10-months before tumor necrosis factor inhibitor (TNFi) initiation and TNFi ineffectiveness, as represented by initiation of a second DMARD after ≥1 month. Models were adjusted for baseline demographic, clinical, treatment, and healthcare-related covariates (see Table) and expressed as adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). HRs >1 signify shorter times to initiation of a second DMARD with antibiotic exposure.
To cite this abstract in AMA style:
Horton D, Verma C, Rege S, Iizuka A, Iozzio M, Koffman D, Crystal S, Davidow A, Gerhard T, Parlett L, Rose C, Strom B. Recent Antibiotic Exposure and Response to Treatment of Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/recent-antibiotic-exposure-and-response-to-treatment-of-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recent-antibiotic-exposure-and-response-to-treatment-of-juvenile-idiopathic-arthritis/