Session Information
Date: Sunday, October 26, 2025
Title: (0357–0386) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Fatigue is a highly prevalent (40%-70%)1 comorbidity in rheumatoid arthritis (RA) patients, and residual fatigue has been described in a significant proportion of patients who achieve low or remission disease activity2.We describe and study the characteristics of fatigued patients compared to non-fatigued using the VAS_Fatigue scale (testing in advance as a good tool), and we assessed residual fatigue according to different activity indices in a group of RA patients.
Methods: RA patients (ACR/EULAR criteria, 2010), excluding Chronic Fatigue Syndrome, were included consecutively in different hospitals across Catalonia (ARcat study group) during 6 months, fulfilling 3 qüestionnaires (PROMs):1. MDHAQ: Function (FN, modified HAQ) (1-10), VAS_Pain (0-10), PGA (0-10), VAS_Fatigue (0-10; fatigue=VAS_Fatigue>53), Review of 60 symptoms (ROS60), self-assessment 48 joint count (sRADAI) and integrated indices: RAPID3 (0-30) for disease activity, FAST3_Pain and FAST3/FAST4_Fatigue for fybromialgia, and MDS2 for depression. 2. FACIT_Fatigue (0-64, indirect score). 3. RAID for impact of the disease (0-10).Sociodemographic and clinical variables: history of depression, disease duration, treatment (DMARDs and glucocorticoids), joint count exam (TJC, SJC: 0-28), acute reactants (ESR, CRP), immunology (RF, ANA) and activity composite indices (DAS28 CRP/ESR, CDAI, SDAI) were assessed. Descriptive and bivariate statistical analyses of variables (quantitatives: Student’s test, categorical: Chi2) comparing fatigued/non-fatigued patients, with a previously Spearman correlation (VAS with FACIT) were performed, and we assessed the frequency of residual fatigue according to disease activity indices.
Results: A total of 246 RA patients (77,1% females) were collected; 61.3±10,5 years, BMI of 26.5±5 kg/m2, disease duration:17.19 (SD:20.39) years and DAS28ESR=3.47 (SD:1.37), treated with glucocorticoids in 52 (37.4%), bDMARD in 63 (45.0%), and Jak inhibitors in 19 (13.6%). A significant level of fatigue (VAS_Fatigue>5) was found in 106 (43.1%) patients with significant differences (p< 0.05) compared to non-fatigued patients in: sex, educational levels, smoking, depression history, disease activity indices, and all of the PROMs scores (Table 1). A strong negative correlation (r=-0.82, p< 0.001) was observed between VAS_Fatigue (0-10) and FACIT_Fatigue (0-54), also good for FN, PN, PATGL, sRADAI, ROS60, RAPID3, RAID, and a moderate for TJC and SJC and composite activity indices, and no correlation for ESR and CRP. A similar level of correlation was obtained for FACIT_Fatigue (Table 2). The presence of residual fatigue (VAS>5) is less frequent (6,3%; p< 0.001) when measuring activity with RAPID3 < 6 than in the rest of the indices. In all of the cases, the differences between the presence or not of fatigue in remission patients were significant (Table 3)
Conclusion: Comorbid fatigue associated with clinical activity, sociodemographics, and PROMs is prevalent in RA patients, and it is feasibly measured by a simple VAS scale included in the MDHAQ. Moreover, the RAPID3 index seems to be the more stringent index to examine residual fatigue. References:1. Hewlett S, 2011. 2. Michaud K, 2020. 3. Tournadre A, 2019.
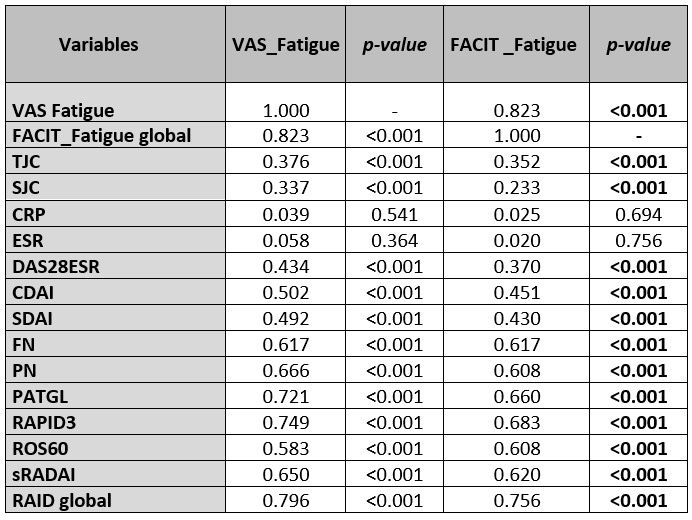 Table 1. Associated factors in fatigued vs non-fatigued patients; only significant differences (p < 0.05) are shown.
Table 1. Associated factors in fatigued vs non-fatigued patients; only significant differences (p < 0.05) are shown.
.jpg) Table 2. Correlation VAS_ Fatiga and FACIT_Fatigue with the clinical study variables.
Table 2. Correlation VAS_ Fatiga and FACIT_Fatigue with the clinical study variables.
.jpg) Table 3. Prevalence of fatigued patients in low activity according to the different activity indices studied; (p < 0.001).
Table 3. Prevalence of fatigued patients in low activity according to the different activity indices studied; (p < 0.001).
To cite this abstract in AMA style:
Morlà Novell R, Frade Sosa B, López-Lasanta M, Sallés Lizarzáburu M, Busquets Pérez N, Salvador Alarcón G, Valls Roc M, Ruiz-Esquide V, Tobalina Mastre L, Sanmartí R, Gomez-Puerta J. Multidimensional Health Assessment Questionnaire (MDHAQ)/RAPID3, is an Useful Tool to Assess Comorbid and Residual Fatigue in Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/multidimensional-health-assessment-questionnaire-mdhaq-rapid3-is-an-useful-tool-to-assess-comorbid-and-residual-fatigue-in-rheumatoid-arthritis-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multidimensional-health-assessment-questionnaire-mdhaq-rapid3-is-an-useful-tool-to-assess-comorbid-and-residual-fatigue-in-rheumatoid-arthritis-patients/
