Session Information
Date: Sunday, October 26, 2025
Title: (0357–0386) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Clinical trials are the cornerstone of evidence-based rheumatology, yet enrolling and retaining a representative patient cohort remains challenging. While underrepresentation of underserved minorities is well documented, patients across all demographic groups may face medical mistrust or logistical barriers that affect trial participation. Prior research using the Thompson et al. (2004) Group-Based Medical Mistrust Scale (GBMMS) has linked higher mistrust to reduced engagement in preventive care, but its impact on rheumatology trial enrollment has not been studied. This study therefore examines whether GBMMS scores predict adult patients’ willingness to enroll in a rheumatology clinical trial and, among those who decline, identifies the logistical concerns that most strongly influence their decision.
Methods: Between November 2024 and February 2025, we conducted a cross-sectional survey in a rheumatology clinic. After informed consent, adults (≥18 years) with physician-confirmed rheumatoid arthritis (2010 ACR/EULAR), systemic lupus erythematosus (2012 SLICC), or other rheumatic conditions completed the 12-item GBMMS (score range 12–60, higher score = more mistrust), 11 logistical-concern items, each rated 1 (“extremely unlikely to influence me”) to 7 (“extremely likely”); and a yes/no question on willingness to participate in a clinical trial. We evaluated GBMMS reliability (Cronbach’s α), compared mean mistrust by willingness status using Welch’s t test, and ran a univariable logistic regression to assess whether mistrust predicted trial willingness. Among those unwilling, we ranked logistical factors by mean importance and calculated Pearson correlations between GBMMS total and each logistical rating (two-tailed α = 0.05).
Results: Forty‑seven patients (79 % women, mean age = 49.23 ± 13.63 years) participated; 66 % reported they would enroll in a clinical trial (Table 1). Internal consistency of the GBMMS was α = 0.90. Mean mistrust scores did not differ among the groups (willing 24.74 ± 11.62 and unwilling 25.25 ± 13.36; Welch’s t(26) = 0.13, p = 0.893) (Table 2) and did not predict willingness in the logistic regression model (OR = 0.996, 95 % CI 0.95 – 1.05, p = 0.89). Among the 16 unwilling to participate in clinical trials, the top-rated logistical factors were: stopping disease progression (mean = 5.38), doctor encouragement (4.56), travel/transportation (4.13) and shared language with doctor (4.13) (Table 3). Across the full sample, mistrust correlated most strongly (though non-significantly) with lower importance on a novel treatment option (r = -0.28; p = 0.056) and doctor encouragement (r = -0.23; p = 0.11).
Conclusion: Group-based medical mistrust did not predict patients’ self-reported willingness to join a clinical trial. Instead, expectation of concrete disease improvement and personal endorsement by one’s treating rheumatologist emerged as the strongest drivers of participation. These findings suggest that recruitment efforts focused on highlighting clear clinical benefits and enlisting rheumatologists to personally invite patients may outperform strategies aimed solely at reducing mistrust.
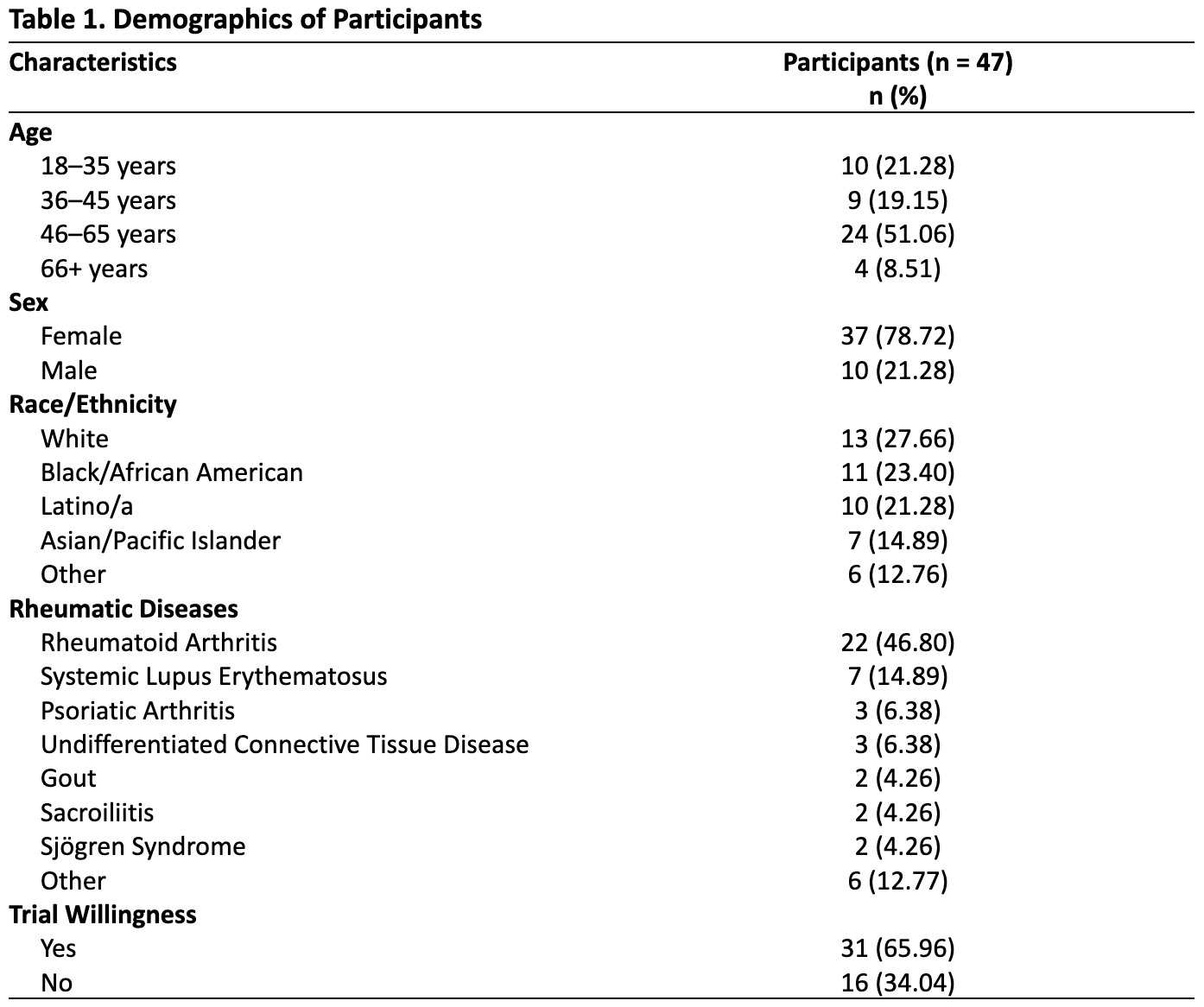 Table 1. Demographics of Participants
Table 1. Demographics of Participants
.jpg?2) Table 2. Mean (SD) Scores on Individual Group-Based Medical Mistrust Scale Items for All Participants and by Willingness to Participate in a Clinical Trial
Table 2. Mean (SD) Scores on Individual Group-Based Medical Mistrust Scale Items for All Participants and by Willingness to Participate in a Clinical Trial
.jpg?3) Table 3. Mean (SD) Importance Ratings of Logistical Factors for All Participants and by Willingness to Participate in a Clinical Trial
Table 3. Mean (SD) Importance Ratings of Logistical Factors for All Participants and by Willingness to Participate in a Clinical Trial
Disclosures: A. Martinez Paulino: None; M. King: None; D. Arias Diaz: None; A. Cheema: None; M. Khawaja: None.
To cite this abstract in AMA style:
Martinez Paulino A, King M, Arias Diaz D, Cheema A, Khawaja M. Group-Based Medical Mistrust and Logistical Factors Influencing Rheumatology Clinical Trial Enrollment: A Single-Center Cross-Sectional Survey [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/group-based-medical-mistrust-and-logistical-factors-influencing-rheumatology-clinical-trial-enrollment-a-single-center-cross-sectional-survey/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/group-based-medical-mistrust-and-logistical-factors-influencing-rheumatology-clinical-trial-enrollment-a-single-center-cross-sectional-survey/
